-
Name
1-Methoxy-2-propanol
- EINECS 203-539-1
- CAS No. 107-98-2
- Article Data103
- CAS DataBase
- Density 0.912 g/cm3
- Solubility soluble in water
- Melting Point - 97 °C
- Formula C4H10O2
- Boiling Point 118.5 °C at 760 mmHg
- Molecular Weight 90.1222
- Flash Point 33.9 °C
- Transport Information UN 3092 3/PG 3
- Appearance colourless liquid
- Safety 26-24/25
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms 1-Methoxy-2-hydroxypropane;Dowtherm 209;Propylene glycol methyl ether;Propylenglykol-monomethylaether;Dowanol-33B;1-methoxypropan-2-ol;.alpha.-Propylene glycol monomethyl ether;2-Methoxy-1-methylethanol;2-Propanol,1-methoxy-;2-Propanol, 1-methoxy-;Propylene Glycol Monomethyl Ether(PM):;Propylene Glycol Monomethyl Ether;Methoxyisopropanol;
- PSA 29.46000
- LogP 0.01360
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine N-oxide; dihydrogen peroxide; methyltrioxorhenium(VII) In methanol; water at 40℃; under 11894.7 - 36201.3 Torr; for 3h; | A 1.02% B 98% |
| With pyridine; carbon dioxide; dihydrogen peroxide; methyltrioxorhenium(VII) In methanol; water at 25 - 40℃; under 36201.3 Torr; for 3h; | A n/a B 60.25% |
| With pyridine N-oxide; carbon dioxide; dihydrogen peroxide; methyltrioxorhenium(VII) In methanol; water at 25 - 40℃; under 36201.3 Torr; for 3 - 9h; | A n/a B 51.1% |
| With pyridine N-oxide; urea hydrogen peroxide adduct; methyltrioxorhenium(VII) In methanol; water at 30℃; under 12929 Torr; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 120℃; for 0.116667h; Concentration; Temperature; Reagent/catalyst; Flow reactor; | 93% |
| With sodium methylate | 78% |
| With Al2O3/MgO composite at 120℃; Inert atmosphere; | 37.4% |
-
-
67-56-1
methanol

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With MCM-41-encapsulated cetyltrimethylammonium hydroxide at 110℃; for 3h; regioselective reaction; | A 92% B n/a |
| With boron trifluoride diethyl etherate | A 36% B 39% |
| at 200℃; |

-
-
187737-37-7
propene

-
A
-
57-55-6
propylene glycol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
1589-47-5
2-methoxypropanol

-
D
-
75-56-9, 16033-71-9
methyloxirane

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; titanium-silicate In methanol at 40℃; | A n/a B n/a C n/a D 92% |
| With dihydrogen peroxide; titanium-silicate In methanol at 40℃; | A n/a B n/a C n/a D 78% |
| With dihydrogen peroxide; titanium-silicate In water at 30 - 80℃; under 18751.9 Torr; pH=4.5; | A n/a B n/a C n/a D 61% |


| Conditions | Yield |
|---|---|
| With iron(III) perchlorate at 20℃; for 24h; neat (no solvent); | 85% |
| With potassium In tetrahydrofuran for 2h; Heating; | 58% |

| Conditions | Yield |
|---|---|
| With Bu3Sn(HMPA)I; tri-n-butyl-tin hydride In tetrahydrofuran at 40℃; for 24h; | 75% |
-
-
187737-37-7
propene

-
A
-
57-55-6
propylene glycol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
75-56-9, 16033-71-9
methyloxirane

| Conditions | Yield |
|---|---|
| With pyridine N-oxide; dihydrogen peroxide; methyltrioxorhenium(VII) In methanol; water at 40℃; under 36201.3 Torr; for 3h; | A 0.59% B 1.85% C 72.73% |
| With dihydrogen peroxide In methanol at 49.84℃; under 5250.53 Torr; Green chemistry; |

-
-
67-56-1
methanol

-
-
124-38-9
carbon dioxide

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
108-32-7
1,2-propylene cyclic carbonate

-
B
-
57-55-6
propylene glycol

-
C
-
107-98-2
1-methoxy-2-propanol

-
D
-
1589-47-5
2-methoxypropanol

-
E
-
616-38-6
carbonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With 6,7,9,10,12,13,20,21-octahydrodibenzo[b,h][1,4,7,10,13,16]hexaoxacyclooctadecine; potassium chloride at 130℃; under 15001.5 Torr; for 8h; Reagent/catalyst; Autoclave; High pressure; | A 69.2% B 12.5% C n/a D n/a E 13.2% |
| With 6,7,9,10,12,13,20,21-octahydrodibenzo[b,h][1,4,7,10,13,16]hexaoxacyclooctadecine; potassium bromide at 130℃; under 15001.5 Torr; for 8h; Reagent/catalyst; Autoclave; High pressure; | A 62.9% B 14.4% C n/a D n/a E 12.5% |
| With potassium bromide at 130℃; under 15001.5 Torr; for 8h; Autoclave; High pressure; | A 44.1% B 6.7% C n/a D n/a E 5.8% |

-
-
67-56-1
methanol

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
1589-47-5
2-methoxypropanol

-
C
-
127-00-4
1-Chloro-2-propanol

| Conditions | Yield |
|---|---|
| With Cu(L-Asp)(1,2-bis(4-pyridyl)ethylene)0.5(H2O)0.5(MeOH)0.5 at 25℃; | A 56% B 40% C 4% |
| With Cu(L-Asp)(1,2-bis(4-pyridyl)ethylene)0.5(H2O)0.5(MeOH)0.5 at 60℃; | A 49% B 47% C 4% |

-
-
67-56-1
methanol

-
-
124-38-9
carbon dioxide

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
108-32-7
1,2-propylene cyclic carbonate

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With lithium iodide at 130℃; under 15001.5 Torr; for 8h; Reagent/catalyst; Autoclave; High pressure; | A 54.8% B n/a C n/a |
| With potassium carbonate at 100℃; for 6h; Supercritical conditions; Green chemistry; | A 35.6% B n/a C n/a |

-
-
67-56-1
methanol

-
-
124-38-9
carbon dioxide

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
108-32-7
1,2-propylene cyclic carbonate

-
B
-
57-55-6
propylene glycol

-
C
-
107-98-2
1-methoxy-2-propanol

-
D
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With lithium bromide at 130℃; under 15001.5 Torr; for 8h; Autoclave; High pressure; | A 52.4% B 5.7% C n/a D n/a |

-
-
186581-53-3, 908094-01-9
diazomethane

-
-
57-55-6
propylene glycol

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With H(+)-zeolite-X In benzene at 25℃; for 0.5h; Product distribution; further alcohols, thiols, aminoalcohols; further catalyst; | A 51% B 33% |
| With H(+)-zeolite-X In benzene at 25℃; for 0.5h; | A 51 % Chromat. B 33 % Chromat. |
| With sulfuric acid In benzene at 25℃; for 0.5h; | A 37 % Chromat. B 38 % Chromat. |


| Conditions | Yield |
|---|---|
| With nickel at 140℃; under 294203 Torr; Hydrogenation; | |
| With hydrogen; In diethylene glycol dimethyl ether; water at 30℃; under 735.5 Torr; for 20h; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride; boron trichloride 1.) CH2Cl2, 10 min, 2.) Et2O, 30 min; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |
-
-
627-40-7
methylallylether

-
A
-
71-23-8
propan-1-ol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
1589-49-7
methoxypropanol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; 9-borabicyclo[3.3.1]nonane dimer; dihydrogen peroxide Product distribution; 1) THF, 25 deg C, 2 h; | A 1.5 % Chromat. B n/a C 94 % Chromat. |


| Conditions | Yield |
|---|---|
| With sodium hydroxide; sodium tetrahydroborate; mercury(II) diacetate 1) water, THF, 30 min, 2) ca. 0.5 h; Yield given. Multistep reaction. Yields of byproduct given; | |
| With sodium hydroxide; mercury(II) diacetate In tetrahydrofuran; water Product distribution; 1) 30 min, 2) ca. 0.5 h; |
-
-
67-56-1
methanol

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
20324-32-7
propylene glycol monomethyl ether dimer

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 85℃; Heating; Yield given. Yields of byproduct given; | |
| With sodium hydroxide at 85℃; Heating; Yield given. Yields of byproduct given; |

-
-
23465-33-0
1-bromo-2-methoxypropane

-
-
7732-18-5
water

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| Purification / work up; Industry scale; |
-
-
187737-37-7
propene

-
A
-
57-55-6
propylene glycol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
74-98-6
propane

-
D
-
1589-47-5
2-methoxypropanol

-
E
-
75-56-9, 16033-71-9
methyloxirane

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; titanium silicate In methanol at 59℃; under 18751.9 Torr; Product distribution / selectivity; |

-
-
67-56-1
methanol

-
-
187737-37-7
propene

-
A
-
57-55-6
propylene glycol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
74-98-6
propane

-
D
-
1589-47-5
2-methoxypropanol

-
E
-
75-56-9, 16033-71-9
methyloxirane

| Conditions | Yield |
|---|---|
| With ammonia; dihydrogen peroxide; titanium-silicate catalyst In water at 41 - 59℃; under 18751.9 Torr; pH=4.5; Product distribution / selectivity; Continuously procces; | |
| With water; hydrogen; oxygen; Pd-Bi/TiO2; tegafur at 60℃; under 16274.9 Torr; for 18h; pH=6; Product distribution / selectivity; aqueous ammonium phosphate buffer; | |
| With water; hydrogen; oxygen; Pd-Au/TiO2; tegafur at 60℃; under 16274.9 Torr; for 18h; pH=6; Product distribution / selectivity; aqueous ammonium phosphate buffer; | |
| With water; hydrogen; oxygen; Pd-Bi-Au/TiO2; tegafur at 60℃; under 16274.9 Torr; for 18h; pH=6; Product distribution / selectivity; aqueous ammonium phosphate buffer; | |
| With ammonium acetate; hydrogen; oxygen at 60℃; under 56255.6 Torr; for 12h; Catalytic behavior; Reagent/catalyst; Temperature; Autoclave; Supercritical conditions; | A n/a B n/a C n/a D n/a E 11.8 %Chromat. |

-
-
7440-32-6
titanium

-
-
187737-37-7
propene

-
-
98-85-1, 13323-81-4
1-Phenylethanol

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In methanol |

-
-
67-56-1
methanol

-
-
187737-37-7
propene

-
B
-
57-55-6
propylene glycol

-
C
-
108-61-2
2,2'-oxydipropanol

-
D
-
107-98-2
1-methoxy-2-propanol

-
E
-
74-98-6
propane

-
F
-
1589-47-5
2-methoxypropanol

-
G
-
75-56-9, 16033-71-9
methyloxirane

| Conditions | Yield |
|---|---|
| With hydrogen; oxygen; Pd/TS-1 In water at 60℃; under 16274.9 Torr; pH=6; Product distribution / selectivity; ammonium phosphate buffer; |

-
-
5878-19-3
Methoxyacetone

-
-
67-63-0
isopropyl alcohol

-
A
-
107-98-2
1-methoxy-2-propanol

-
B
-
67-64-1
acetone

| Conditions | Yield |
|---|---|
| With Tri-tert-butylaluminium; trifluoroacetic acid In toluene at 60℃; for 24h; Meerwein-Ponndorf-Verley reduction; Inert atmosphere; |

-
-
7778-85-0
1,2-dimethoxypropane

-
A
-
74-87-3
methylene chloride

-
B
-
26198-63-0, 78-87-5
1,2-Dichloropropane

-
C
-
107-98-2
1-methoxy-2-propanol

-
D
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| Stage #1: 1,2-dimethoxypropane With dichloromethane; tungsten(VI) chloride In Chloroform-D at 100℃; for 3h; Stage #2: With water In Chloroform-D at -20℃; |

-
-
67-56-1
methanol

-
-
124-38-9
carbon dioxide

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
108-32-7
1,2-propylene cyclic carbonate

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
616-38-6
carbonic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With Br(1-)*C14H33N2(1+) at 170℃; under 15001.5 - 37503.8 Torr; for 8h; Autoclave; |

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
57-55-6
propylene glycol

-
B
-
107-98-2
1-methoxy-2-propanol

-
C
-
1589-47-5
2-methoxypropanol

| Conditions | Yield |
|---|---|
| With 0.5% Pt/Al2O3; dihydrogen peroxide at 59.84℃; under 3750.38 Torr; for 2h; Inert atmosphere; Autoclave; |

-
-
107-98-2
1-methoxy-2-propanol

-
-
124-63-0
methanesulfonyl chloride

-
-
24590-51-0
(+/-)-(methoxyprop-2-yl) methanesulphonate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; | 100% |
| With pyridine at -5℃; |
-
-
936247-35-7
5-chloro-2-nitropyridin-3-ol

-
-
107-98-2
1-methoxy-2-propanol

-
-
1144110-19-9
5-chloro-3-(2-methoxy-1-methyl-ethoxy)-2-nitro-pyridine

| Conditions | Yield |
|---|---|
| With di-tert-butyl-diazodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; for 5h; | 100% |
-
-
107-98-2
1-methoxy-2-propanol

-
-
700-36-7
2-bromo-4-fluoronitrobenzene

-
-
943247-79-8
2-bromo-4-(2-methoxy-1-methylethoxy)-1-nitrobenzene

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-2-propanol With sodium hydride In N,N-dimethyl-formamide at 0℃; for 0.5h; Stage #2: 2-bromo-4-fluoronitrobenzene In N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 100% |
-
-
17464-88-9
1,3,4,6-tetrakis(methoxymethyl)glycoluril

-
-
107-98-2
1-methoxy-2-propanol

| Conditions | Yield |
|---|---|
| With Amberlyst 15 resin at 60℃; for 17h; Catalytic behavior; Reagent/catalyst; | 99.8% |
| With AMBERLYST at 25 - 60℃; under 80 - 100 Torr; for 7h; |

| Conditions | Yield |
|---|---|
| 99.4% | |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; trichloroisocyanuric acid; sodium hydrogencarbonate In dichloromethane at 0 - 10℃; for 3.5h; Temperature; | 97.8% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; chlorine; sodium carbonate In dichloromethane at 0 - 10℃; for 3.5h; Reagent/catalyst; Temperature; | 97.6% |
-
-
107-98-2
1-methoxy-2-propanol

-
-
61706-54-5
N-(chloromethyl)-N-(2,6-dimethylphenyl)-α-chloroacetamide

-
-
83447-95-4
2',6'-Dimethyl-N-(2-Methoxy-1-Methylethoxymethyl) 2-Chloroacetanilide

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane | 99.1% |

-
-
107-98-2
1-methoxy-2-propanol

-
-
106-89-8
epichlorohydrin

-
-
62-23-7
4-nitro-benzoic acid

-
-
60827-45-4
(S)-3-chloropropan-1,2-diol

| Conditions | Yield |
|---|---|
| In water | 99% |


| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In toluene at 50℃; for 2h; Temperature; Cooling with ice; | 99% |
| With di-isopropyl azodicarboxylate; triphenylphosphine In toluene at 50℃; for 2h; Cooling with ice; | 62 %Chromat. |
| With di-isopropyl azodicarboxylate; triphenylphosphine In toluene at 50℃; for 2h; Cooling with ice; | 62 %Chromat. |

| Conditions | Yield |
|---|---|
| With ytterbium(III) triflate at 140℃; for 20h; | 97% |

| Conditions | Yield |
|---|---|
| With zirconium(IV) oxide at 100℃; for 3h; Temperature; Reagent/catalyst; | 96.6% |

| Conditions | Yield |
|---|---|
| With dmap In 2-methoxy-ethanol | 96% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 95.3% |

-
-
107-98-2
1-methoxy-2-propanol

-
-
179898-34-1
3-bromo-5-fluorobenzonitrile

-
-
883907-45-7
3-bromo-5-(2-methoxy-1-methylethoxy)benzonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-methoxy-2-propanol With sodium hexamethyldisilazane In N,N-dimethyl-formamide at 25℃; for 0.25h; Stage #2: 3-bromo-5-fluorobenzonitrile In N,N-dimethyl-formamide at 23 - 27℃; for 0.583333h; | 95% |

| Conditions | Yield |
|---|---|
| In pentane byproducts: CH4; Ar-atmosphere; dropwise addn. of ligand to AlMe3 at -78°C, stirring for 2 h, then at room temp. for 12 h; solvent removal (vac.), sublimation (110°C/0.1 mbar); elem. anal.; | 94% |
-
-
13734-41-3
t-Boc-L-valine

-
-
107-98-2
1-methoxy-2-propanol

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 94% |
-
-
107-98-2
1-methoxy-2-propanol

| Conditions | Yield |
|---|---|
| With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran; dichloromethane at 0 - 20℃; Mitsunobu Displacement; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| In pentane byproducts: CH4; Ar-atmosphere; dropwise addn. of ligand to GaMe3 at room temp., stirringovernight; distn. off of solvent, sublimation (74°C/0.1 mbar), then distn. (50°C/0.1 mbar) and crystn. on standing at -30°C for 1 h; elem. anal.; | 89% |
-
-
17228-64-7
6-chloro-2-methoxypyridine

-
-
107-98-2
1-methoxy-2-propanol

| Conditions | Yield |
|---|---|
| With C34H47ClNiO3P2; sodium t-butanolate In toluene at 110℃; for 18h; Inert atmosphere; Sealed tube; | 89% |
-
-
107-98-2
1-methoxy-2-propanol

-
-
34306-42-8
Boc-Abu

| Conditions | Yield |
|---|---|
| With dmap; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 88% |
-
-
107-98-2
1-methoxy-2-propanol

-
-
1191102-65-4
ethyl 7-hydroxy-5-[4-(methylsulfonyl)phenoxy]-1H-indole-2-carboxylate

-
-
1191103-54-4
ethyl 7-(2-methoxy-1-methylethoxy)-5-[4-(methylsulfonyl)phenoxy]-1H-indole-2-carboxylate

| Conditions | Yield |
|---|---|
| With tributylphosphine; 1,1'-azodicarbonyl-dipiperidine In tetrahydrofuran at 50℃; | 87% |
-
-
292638-84-7
styrene

-
-
107-98-2
1-methoxy-2-propanol

| Conditions | Yield |
|---|---|
| With tris[2-phenylpyridinato-C2,N]iridium(III) In dichloromethane at 20℃; for 2.5h; Schlenk technique; Inert atmosphere; Irradiation; regioselective reaction; | 87% |

-
-
107-98-2
1-methoxy-2-propanol

-
-
372-09-8
cyanoacetic acid

-
-
32804-79-8
1-methoxypropan-2-yl 2-cyanoacetate

| Conditions | Yield |
|---|---|
| With sulfuric acid In benzene for 2.5h; Fischer esterification; Heating; | 85% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; CO2; potassium carbonate In water | 85% |
-
-
107-98-2
1-methoxy-2-propanol

-
-
77111-77-4
2-chloro-9-(tetrahydro-2H-pyran-2-yl)-9H-purin-6-amine

-
-
1050495-05-0
2-([1-methyl-2-(methoxy)ethyl]oxy)-9-(tetrahydro-2H-pyran-2-yl)-9H-purin-6-amine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 80 - 90℃; for 2h; | 84.7% |
1-Methoxy-2-propanol Specification
The CAS registry number of Methoxy propanol is 107-98-2. The IUPAC name is 1-methoxypropan-2-ol. In addition, the molecular formula is C4H10O2. What's more, it is a kind of colourless liquid and incompatible with strong oxidizing agents, acid chlorides, acid anhydrides, water. It is mainly used as a solvent, dispersant and diluent. Besides, it is also used as antifreeze agent for the fuel, extraction solvent.
Physical properties about this chemical are: (1)ACD/LogP: -0.45; (2)ACD/LogD (pH 5.5): -0.45; (3)ACD/LogD (pH 7.4): -0.45; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 13.56; (7)ACD/KOC (pH 7.4): 13.56; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 18.46 Å2; (12)Index of Refraction: 1.397; (13)Molar Refractivity: 23.81 cm3; (14)Molar Volume: 98.8 cm3; (15)Polarizability: 9.44 ×10-24cm3; (16)Surface Tension: 26.9 dyne/cm; (17)Density: 0.912 g/cm3; (18)Flash Point: 33.9 °C; (19)Enthalpy of Vaporization: 41.58 kJ/mol; (20)Boiling Point: 118.5 °C at 760 mmHg; (21)Vapour Pressure: 8.15 mmHg at 25°C.
Preparation of Methoxy propanol: it can be prepared by methyloxirane and methanol. This reaction will need reagent NaOCH3. The yield is about 78%.
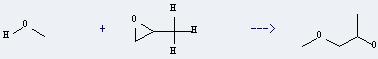
Uses of Methoxy propanol: it can react with oxirane to get 4-methyl-2,5-dioxaheptan-7-ol. This reaction will need reagent BF3*Et2O. The yield is about 53%.
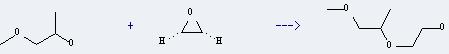
When you are using this chemical, please be cautious about it as the following:
This chemical is flammable. During using it, you should avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: OC(C)COC
(2)InChI: InChI=1/C4H10O2/c1-4(5)3-6-2/h4-5H,3H2,1-2H3
(3)InChIKey: ARXJGSRGQADJSQ-UHFFFAOYAJ
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LD50 | intravenous | 2gm/kg (2000mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. |
| dog | LD50 | oral | 5gm/kg (5000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. | |
| guinea pig | LCLo | inhalation | 15000ppm/7H (15000ppm) | BEHAVIORAL: SLEEP | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 9, Pg. 509, 1954. |
| human | TCLo | inhalation | 3000ppm (3000ppm) | SENSE ORGANS AND SPECIAL SENSES: TUMORS: OLFACTION BEHAVIORAL: GENERAL ANESTHETIC GASTROINTESTINAL: NAUSEA OR VOMITING | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 105, 1974. |
| mouse | LD | intraperitoneal | > 500mg/kg (500mg/kg) | "Summary Tables of Biological Tests," National Research Council Chemical-Biological Coordination Center. Vol. 4, Pg. 110, 1952. | |
| mouse | LD50 | intravenous | 5300mg/kg (5300mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. |
| mouse | LD50 | oral | 11700mg/kg (11700mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. |
| rabbit | LCLo | inhalation | 15000ppm/7H (15000ppm) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 9, Pg. 509, 1954. | |
| rabbit | LD50 | intravenous | 1200mg/kg (1200mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. | |
| rabbit | LD50 | oral | 5700mg/kg (5700mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. | |
| rabbit | LD50 | skin | 13gm/kg (13000mg/kg) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 105, 1974. | |
| rabbit | LD50 | subcutaneous | 5gm/kg (5000mg/kg) | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. | |
| rat | LC50 | inhalation | 10000ppm/5H (10000ppm) | Raw Material Data Handbook, Vol.1: Organic Solvents, 1974. Vol. 1, Pg. 105, 1974. | |
| rat | LD50 | intraperitoneal | 3720mg/kg (3720mg/kg) | "Patty's Industrial Hygiene and Toxicology," 3rd rev. ed., Clayton, G.D., and F.E. Clayton, eds., New York, John Wiley & Sons, Inc., 1978-82. Vol. 3 originally pub. in 1979; pub. as 2n rev. ed. in 1985.Vol. 2C, Pg. 3977, 1982. | |
| rat | LD50 | intravenous | 4200mg/kg (4200mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: ATAXIA LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. |
| rat | LD50 | subcutaneous | 7800mg/kg (7800mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: DYSPNEA BEHAVIORAL: ATAXIA | Arzneimittel-Forschung. Drug Research. Vol. 22, Pg. 569, 1972. |
| rat | LDLo | oral | 3739mg/kg (3739mg/kg) | SENSE ORGANS AND SPECIAL SENSES: CHROMODACYRORREA: EYE BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) BEHAVIORAL: ATAXIA | National Technical Information Service. Vol. OTS0520402, |
Related Products
- 1-Methoxy-2-propanol
- 107983-78-8
- 107990-50-1
- 107991-51-5
- 107-99-3
- 1079950-08-5
- 1079950-10-9
- 10-80-0
- 1080-06-4
- 108-00-9
- 108-01-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View