-
Name
1-PROPOXY-2-PROPANOL
- EINECS 250-069-8
- CAS No. 1569-01-3
- Article Data22
- CAS DataBase
- Density 0.885 g/mL at 25 °C(lit.)
-
Solubility
miscible
- Melting Point -80 C
- Formula C6H14 O2
- Boiling Point 140 - 160 C
- Molecular Weight 118.176
- Flash Point 48 C
- Transport Information HAZARD
- Appearance clear liquid
- Safety
- Risk Codes R10; R36/37/38
- Molecular Structure
- Hazard Symbols UN NO.
- Synonyms 1,2-Propyleneglycol 1-propyl ether; 1-Propoxy-2-propanol; Arcosolv PNP
- PSA 29.46000
- LogP 0.79380
Synthetic route
Conditions
Conditions Yield With Al2O3/MgO composite at 120℃; Inert atmosphere; 34.4% With KAcMIMOH at 60℃; for 4h; With hematite at 160℃; for 8h; Autoclave; -
-
71-23-8
propan-1-ol

-
-
75-56-9, 16033-71-9
methyloxirane

-
A
-
10215-30-2
2-propoxy-1-propanol

-
B
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield at 100 - 200℃; at 180 - 205℃; With sulfuric acid Yield given. Yields of byproduct given; ...Expand
-
-
106-92-3
Allyl glycidyl ether

-
A
-
3126-95-2
3-Propoxy-1,2-epoxypropan

-
B
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield With hydrogen; 5percent Pd/C(en) In tetrahydrofuran at 20℃; for 2.5h; Catalytic hydrogenation; Title compound not separated from byproducts.; -
-
57-55-6
propylene glycol

-
A
-
71-23-8
propan-1-ol

-
B
-
111-43-3
Dipropyl ether

-
C
-
10215-30-2
2-propoxy-1-propanol

-
D
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield With trifluorormethanesulfonic acid; hydrogen; [{CpRu(CO)2}2(μ-H)](+)*OTf(-); cyclopenta-1,3-diene In sulfolane at 110℃; under 39003.1 Torr; for 30h; Product distribution; A 54 % Chromat.
B 15 % Chromat.
C n/a
D n/a...Expand
-
-
57-55-6
propylene glycol

-
A
-
71-23-8
propan-1-ol

-
B
-
4359-46-0
2-ethyl-4-methyl-1,3-dioxolane

-
C
-
111-43-3
Dipropyl ether

-
D
-
10215-30-2
2-propoxy-1-propanol

-
E
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
F
-
123-38-6
propionaldehyde
Conditions
Conditions Yield With bis(dicarbonyl)(μ-hydrido)(pentamethylcyclopentadiene)ruthenium trifluoromethanesulfonate; trifluorormethanesulfonic acid; hydrogen In sulfolane at 110℃; under 38787.1 Torr; for 30h; Mechanism; Kinetics; Solvent; Pressure; Concentration; Temperature; Time; regioselective reaction; A 54 %Chromat.
B n/a
C n/a
D n/a
E n/a
F n/a...Expand
-
-
57-55-6
propylene glycol

-
A
-
71-23-8
propan-1-ol

-
B
-
106-36-5
propyl propionate

-
C
-
74-98-6
propane

-
D
-
10215-30-2
2-propoxy-1-propanol

-
E
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
F
-
802294-64-0
propionic acid

-
G
-
108-67-8
1,3,5-trimethyl-benzene
Conditions
Conditions Yield With [C6H3-2,6-(OP(tBu)2)2]IrH2; trifluorormethanesulfonic acid; hydrogen In 1,4-dioxane at 125℃; under 5171.62 Torr; Autoclave; ...Expand Conditions
Conditions
Conditions Yield With water at 80℃; for 24h; Equilibrium constant; Sealed tube; -
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
-
1056233-21-6
N’-(5-bromo-6-hydroxy-2-methyl-3-pyridyl)-N-ethyl-N-methyl-formamidine
Conditions
Conditions Yield With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20 - 40℃; for 1.66667h; Inert atmosphere; 95% With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 20℃; for 1.5h; Inert atmosphere; 95% Conditions
Conditions Yield With [bis({2‐[bis(propan‐2‐yl)phosphanyl]ethyl})amine](borohydride)(carbonyl)(hydride)iron(II); methacrylic acid methyl ester In toluene at 140℃; under 13689.1 Torr; for 4h; Autoclave; Inert atmosphere; 71% -
-
874493-25-1
3-bromo-6-methyl-5-nitro-pyridin-2-ol

-
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 40 - 65℃; for 12h; Inert atmosphere; 60% With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 65℃; for 12h; Inert atmosphere; 60% -
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 40 - 60℃; for 25h; Inert atmosphere; 53% With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 60℃; for 25h; Inert atmosphere; 53% -
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
-
68058-77-5
1-propoxy-propan-2-one
Conditions
Conditions Yield With sodium dichromate; sulfuric acid at 4 - 5℃; Conditions
Conditions Yield at 4 - 5℃; ...Expand
-
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield Multi-step reaction with 2 steps
1: Na2Cr2O7; diluted H2SO4 / 4 - 5 °C
View Scheme-
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
-
616-38-6
carbonic acid dimethyl ester

-
A
-
4188-67-4
propylpropylene glycol(2) methyl ether

-
B
-
1220964-45-3
C8H16O4
Conditions
Conditions Yield With sodium methylate at 90℃; under 750.075 Torr; for 20h; Inert atmosphere; A 9 %Chromat.
B 91 %Chromat....Expand
-
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield Multi-step reaction with 2 steps
1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 40 - 65 °C / Inert atmosphere
2: ammonium chloride; iron / ethanol; water / 14 h / 85 °C
View SchemeMulti-step reaction with 2 steps
1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran / 12 h / 65 °C / Inert atmosphere
2: ammonium chloride; iron / ethanol; water / 14 h / 85 °C
View Scheme-
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield Multi-step reaction with 3 steps
1.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 40 - 65 °C / Inert atmosphere
2.1: ammonium chloride; iron / ethanol; water / 14 h / 85 °C
3.1: trichlorophosphate / dichloromethane / 1 h / 20 °C
3.2: 2 h / 20 °C
View SchemeMulti-step reaction with 3 steps
1.1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran / 12 h / 65 °C / Inert atmosphere
2.1: ammonium chloride; iron / ethanol; water / 14 h / 85 °C
3.1: trichlorophosphate / dichloromethane / 1 h / 20 °C
3.2: 2 h / 20 °C
View Scheme-
-
1569-01-3
1-(1-propyloxy)propan-2-ol
Conditions
Conditions Yield Multi-step reaction with 3 steps
1.1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 12 h / 40 - 65 °C / Inert atmosphere
2.1: ammonium chloride; iron / ethanol; water / 14 h / 85 °C
3.1: trichlorophosphate / dichloromethane / 1 h / 20 °C
3.2: 2 h / 20 °C
View Scheme-
-
1569-01-3
1-(1-propyloxy)propan-2-ol

-
-
1056233-21-6
N’-(5-bromo-6-hydroxy-2-methyl-3-pyridyl)-N-ethyl-N-methyl-formamidine
Conditions
Conditions Yield Multi-step reaction with 2 steps
1: triphenylphosphine; di-isopropyl azodicarboxylate / tetrahydrofuran / 1.67 h / 20 - 40 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 18 h / 120 °C / Inert atmosphere
View SchemeMulti-step reaction with 2 steps
1: di-isopropyl azodicarboxylate; triphenylphosphine / tetrahydrofuran / 1.5 h / 20 °C / Inert atmosphere
2: tetrakis(triphenylphosphine) palladium(0) / N,N-dimethyl-formamide / 18 h / 120 °C / Inert atmosphere
View SchemeConditions
Conditions Yield at 80℃; for 24h; Equilibrium constant; Sealed tube; 1-PROPOXY-2-PROPANOL Chemical Properties
Synonyms: PNP;2-Propanol, 1-propoxy-;PROPYLENE GLYCOL PROPYL ETHER;PROPYLENE GLYCOL MONOPROPYL ETHER;PROPYLENE GLYCOL NORMAL PROPYL ETHER;ethermonopropyliquenormaldupropyleneglycol ;1-propoxy-2-propano;1-propoxy-propan-2-ol;
The Molecular formula of 1-PROPOXY-2-PROPANOL(1569-01-3): C6H14O2
The Molecular Weight of 1-PROPOXY-2-PROPANOL(1569-01-3): 118.17
Molecular Structure :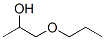
EINECS: 216-372-4
Boiling point: 140-160 °C(lit.)
Flash point: 119 °F
density: 0.885 g/mL at 25 °C(lit.)
refractive index: n20/D 1.411(lit.)1-PROPOXY-2-PROPANOL Toxicity Data With Reference
1. skn-rbt 500 mg
VHTODE Veterinary and Human Toxicology. 30 (1988),126. 2. eye-rbt 100 mg MOD
VHTODE Veterinary and Human Toxicology. 30 (1988),126. 3. orl-rat LD50:2504 mg/kg
VHTODE Veterinary and Human Toxicology. 30 (1988),126. 4. skn-rbt LD50:3550 mg/kg
38MKAJ Pattys Industrial Hygiene and Toxicology. 2C (1982),3983. 1-PROPOXY-2-PROPANOL Consensus Reports
Reported in EPA TSCA Inventory.1-PROPOXY-2-PROPANOL Safety Profile
Moderately toxic by ingestion and skin contact. A skin and eye irritant. When heated to decomposition it emits acrid smoke and irritating fumes.
The Hazard Codes of 1-PROPOXY-2-PROPANOL(1569-01-3): Xi
Xi
The Risk Statements information of 1-PROPOXY-2-PROPANOL(1569-01-3):
10: Flammable
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 1-PROPOXY-2-PROPANOL(1569-01-3):
16: Keep away from sources of ignition - No smoking
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
RIDADR: UN 3271 3/PG 3Related Products
- 1-PROPOXY-2-PROPANOL
- 156901-58-5
- 1569-02-4
- 15690-24-1
- 15690-25-2
- 15690-38-7
- 156912-12-8
- 1569-16-0
- 156917-23-6
- 156925-22-3
- 156925-25-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View