-
Name
19-Norandrost-5(10)-ene-3,17-dione
- EINECS 223-564-1
- CAS No. 3962-66-1
- Article Data26
- CAS DataBase
- Density 1.13 g/cm3
- Solubility 24mg/L at 20℃
- Melting Point 144-146 °C
- Formula C18H24O2
- Boiling Point 427.5 °C at 760 mmHg
- Molecular Weight 272.387
- Flash Point 159.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Estr-5(10)-ene-3,17-dione;
- PSA 34.14000
- LogP 3.84140
Synthetic route

| Conditions | Yield |
|---|---|
| With pyridine for 0.5h; Heating; | 82% |
| In pyridine at 50℃; for 1h; | 700 mg |
-
-
968-49-0
19-oxoandrostenedione

-
A
-
3962-66-1
estra-5(10)-en-3,17-dione

-
C
-
160617-57-2
(19R)-19-hydroxy-5β,19-cycloandrostane-3,17-dione

-
D
-
160617-58-3
(19S)-19-hydroxy-5β,19-cycloandrostane-3,17-dione

| Conditions | Yield |
|---|---|
| With acetic acid; zinc for 1.5h; Title compound not separated from byproducts; | A 3.3% B n/a C 62.5% D n/a |
| With acetic acid; zinc for 1.5h; | A 3.3% B n/a C 62.5% D n/a |

-
-
968-49-0
19-oxoandrostenedione

-
A
-
1089-78-7
17β-hydroxy-estr-5(10)-en-3-one

-
B
-
3962-66-1
estra-5(10)-en-3,17-dione

-
C
-
160617-57-2
(19R)-19-hydroxy-5β,19-cycloandrostane-3,17-dione

-
D
-
192373-63-0
(19R)-17β,19-dihydroxy-5β,19-cycloandrostan-3-one

| Conditions | Yield |
|---|---|
| With ammonia; lithium In tetrahydrofuran for 0.5h; | A 12% B 11% C 11% D 34% |

-
-
17976-32-8
3-methoxyestra-2,5(10)-dien-17-one

-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| With methanol; acetic acid |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 60 percent / pyridinium dichromate / CH2Cl2 / 18 h / 20 °C 2: 3.3 percent / Zn, 50percent aq. AcOH / 1.5 h View Scheme | |
| Multi-step reaction with 2 steps 1: 7.3 g / Cr2O3, conc. H2SO4 / acetone / 0.5 h / 10 - 15 °C 2: 700 mg / pyridine / 1 h / 50 °C View Scheme |

| Conditions | Yield |
|---|---|
| With malonic acid In water; acetone at 20℃; for 4h; Temperature; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: potassium carbonate / N,N-dimethyl-formamide / 16 h / 130 °C 2.1: orthoformic acid triethyl ester; boron trifluoride diethyl etherate / 0.25 h / 25 °C 2.2: 5 h / 25 °C 3.1: ammonia; sodium hydroxide; lithium / tetrahydrofuran / 0.5 h / -78 - -50 °C 3.2: 1 h 4.1: malonic acid / acetone; water / 4 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: orthoformic acid triethyl ester; boron trifluoride diethyl etherate / 0.25 h / 25 °C 1.2: 5 h / 25 °C 2.1: ammonia; sodium hydroxide; lithium / tetrahydrofuran / 0.5 h / -78 - -50 °C 2.2: 1 h 3.1: malonic acid / acetone; water / 4 h / 20 °C View Scheme |
-
-
28336-29-0
17,17-ethylenedioxy-3-methoxyoestra-1,3,5(10)-triene

-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: ammonia; sodium hydroxide; lithium / tetrahydrofuran / 0.5 h / -78 - -50 °C 1.2: 1 h 2.1: malonic acid / acetone; water / 4 h / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate); triphenylphosphine; N,N,N',N'-tetramethylguanidine In tetrahydrofuran at 20℃; for 17h; regioselective reaction; | 56% |
| (i) Na2CrO4, AcOH, (ii) SiO2; Multistep reaction; |
-
-
93-59-4
Perbenzoic acid

-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
5189-96-8
10β-hydroxy-19-norandrost-4-ene-3,17-dione

| Conditions | Yield |
|---|---|
| With benzene |

| Conditions | Yield |
|---|---|
| With malonic acid for 19h; | 5.1 g |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
107-21-1
ethylene glycol

-
A
-
6193-99-3
3,3-(ethylenedioxy)-5(10)-estren-17-one

-
B
-
2220-74-8
estr-5(10)-ene-3,17-dione bis(ethylene ketal)

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene for 2h; Heating; | A 586 mg B 2.55 g |

-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
107-21-1
ethylene glycol

-
-
2220-74-8
estr-5(10)-ene-3,17-dione bis(ethylene ketal)

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In benzene Heating; Yield given; |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
813-77-4
Dimethyl chlorophosphate

-
-
118544-10-8
Phosphoric acid dimethyl ester (8R,9S,13S,14S)-13-methyl-17-oxo-2,6,7,8,9,11,12,13,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl ester

| Conditions | Yield |
|---|---|
| With 2,2,6,6-tetramethyl-piperidine; n-butyllithium 1.) hexane, THF, -70 deg C, 15 min, to room temp.; 2.) HMPA, 5 min, room temp.; Yield given. Multistep reaction; |

| Conditions | Yield |
|---|---|
| (enzymatic reduction); |

| Conditions | Yield |
|---|---|
| With dioxyphthalic acid In diethyl ether; chloroform for 2h; Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2.55 g / TsOH / benzene / 2 h / Heating 2: 1.) tBuOK, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, TsOH, 2 h View Scheme | |
| Multi-step reaction with 2 steps 1: 2.55 g / TsOH / benzene / 2 h / Heating 2: 1.) tBuOK, CHBr3, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, TsOH, 2 h View Scheme | |
| Multi-step reaction with 5 steps 1: p-TSA / benzene / Heating 2: N-bromosuccinimide, MgO, H2O / dimethylformamide / 2 h / Ambient temperature 3: 71 percent / NaOMe / methanol; tetrahydrofuran / 8 h / 4 °C 4: 71 percent / KHS, 18-crown-6 / ethane-1,2-diol / 2 h / 140 °C 5: 27 percent / p-TSA / acetone / Ambient temperature View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
139042-19-6
17β-((tert-Butyldimethylsilyl)oxy)estr-4-en-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) aq. NaOH, BTEAC, CHCl3, 2.) TsOH / 1.) room temperature, 16 h, 2.) acetone, 30 min View Scheme | |
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 1.) tBuOK, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, 2 h View Scheme | |
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 1.) tBuOK, CHBr3, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, 2 h View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2.55 g / TsOH / benzene / 2 h / Heating 2: 1.) tBuOK, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, TsOH, 2 h View Scheme | |
| Multi-step reaction with 2 steps 1: 2.55 g / TsOH / benzene / 2 h / Heating 2: 1.) tBuOK, CHBr3, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, TsOH, 2 h View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) aq. NaOH, BTEAC, CHCl3, 2.) TsOH / 1.) room temperature, 16 h, 2.) acetone, 30 min View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
158388-33-1
tert-Butyl-((8R,9S,13S,14S,17S)-3,3-dimethoxy-13-methyl-2,3,4,6,7,8,9,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-17-yloxy)-dimethyl-silane

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
158388-29-5
17β-(tert-butyldimethylsiloxy)-3,3-ethylenedioxyestr-5(10)-ene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) CTAB, aq. NaOH, 2.) TsOH / 1.) 48 h, 2.) acetone, 1 h View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) aq. NaOH, BTEAC, CHCl3, 2.) TsOH / 1.) room temperature, 16 h, 2.) acetone, 30 min View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) CTAB, aq. NaOH, 2.) aq. HCl / 1.) 18 h, 2.) acetone, 30 min View Scheme | |
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 1.) tBuOK, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, 2 h View Scheme | |
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 1.) tBuOK, CHBr3, 2.) TsOH / 1.) Et2O, -30 deg C, 24 h, 2.) acetone, 2 h View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) aq. NaOH, BTEAC, CHCl3, 2.) TsOH / 1.) room temperature, 16 h, 2.) acetone, 30 min View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 650 mg / CTAB, aq. NaOH / 18 h / Ambient temperature View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 41 mg / various solvent(s) / 3.5 h / 120 - 130 °C View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 586 mg / TsOH / benzene / 2 h / Heating 2: 1.) NaBH4, 2.) imidazole / 1.) MeOH, 1 h, 2.) DMF, 50 deg C, 2 h 3: 1.) aq. NaOH, CTAB, 2.) TsOH / 1.) 18 h, 2.) acetone View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
158388-34-2
19(S)-bromo-17β-(tert-butyldimethylsiloxy)-5β,6β-dibromomethylene-9α,19-cyclo-10α-androstan-3-one

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) CTAB, aq. NaOH, 2.) TsOH / 1.) 48 h, 2.) acetone, 1 h View Scheme | |
| Multi-step reaction with 4 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 1.) CTAB, aq. NaOH, 2.) aq. HCl / 1.) 18 h, 2.) acetone, 30 min View Scheme | |
| Multi-step reaction with 5 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h 3: 1.7 g / imidazole / dimethylformamide / 1 h / 50 °C 4: 650 mg / CTAB, aq. NaOH / 18 h / Ambient temperature 5: 200 mg / aq. HCl / acetone / 0.5 h / Ambient temperature View Scheme |
-
-
3962-66-1
estra-5(10)-en-3,17-dione

-
-
56016-36-5
3,3-dimethoxyestr-5(10)-en-17β-ol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 5.1 g / malonic acid / 19 h 2: 5.2 g / NaBH4 / methanol / 1 h View Scheme |
19-Norandrost-5(10)-ene-3,17-dione Chemical Properties
Molecule structure of 19-Norandrost-5(10)-ene-3,17-dione (CAS NO.3962-66-1):
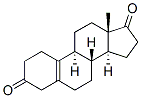
IUPAC Name: (8R,9S,13S,14S)-13-Methyl-1,2,4,6,7,8,9,11,12,14,15,16-dodecahydrocyclopenta[a]phenanthrene-3,17-dione
Molecular Weight: 272.38196 g/mol
Molecular Formula: C18H24O2
Density: 1.13 g/cm3
Boiling Point: 427.5 °C at 760 mmHg
Flash Point: 159.7 °C
Index of Refraction: 1.555
Molar Refractivity: 76.85 cm3
Molar Volume: 239 cm3
Surface Tension: 43.4 dyne/cm
Enthalpy of Vaporization: 68.25 kJ/mol
Vapour Pressure: 1.63E-07 mmHg at 25 °C
XLogP3-AA: 1.9
H-Bond Acceptor: 2
Tautomer Count: 16
Exact Mass: 272.17763
MonoIsotopic Mass: 272.17763
Topological Polar Surface Area: 34.1
Heavy Atom Count: 20
Canonical SMILES: CC12CCC3C(C1CCC2=O)CCC4=C3CCC(=O)C4
Isomeric SMILES: C[C@]12CC[C@H]3[C@H]([C@@H]1CCC2=O)CCC4=C3CCC(=O)C4
InChI: InChI=1S/C18H24O2/c1-18-9-8-14-13-5-3-12(19)10-11(13)2-4-15(14)16(18)6-7-17(18)20/h14-16H,2-10H2,1H3/t14-,15-,16+,18+/m1/s1
InChIKey: RORYLAUXKSWMQL-CBZIJGRNSA-N
EINECS: 223-564-1
Product Categories: Steroids
19-Norandrost-5(10)-ene-3,17-dione Uses
19-Norandrost-5(10)-ene-3,17-dione (CAS NO.3962-66-1) is used in pharmaceutical synthesis.
19-Norandrost-5(10)-ene-3,17-dione Specification
19-Norandrost-5(10)-ene-3,17-dione (CAS NO.3962-66-1) is also named as Oestr-5(10)-ene-3,17-dione .
Related Products
- 19-Norandrost-5(10)-ene-3,17-dione
- 39627-61-7
- 3962-77-4
- 39627-84-4
- 39627-98-0
- 39630-25-6
- 396-30-5
- 3963-45-9
- 39635-11-5
- 39635-32-0
- 39635-79-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View