-
Name
2,2,4-Trimethylpentane
- EINECS 208-759-1
- CAS No. 540-84-1
- Article Data123
- CAS DataBase
- Density 0.692 g/cm3
- Solubility insoluble in water
- Melting Point -107 °C
- Formula C8H18
- Boiling Point 98.8 °C at 760 mmHg
- Molecular Weight 114.231
- Flash Point 18°F
- Transport Information UN 1262
- Appearance colourless liquid
- Safety 9-16-29-33-60-61-62
- Risk Codes 11-38-50/53-65-67
-
Molecular Structure
-
Hazard Symbols
 F,
F,  Xn,
Xn,  N
N
- Synonyms Isobutyltrimethylmethane;NSC 39117;iso-Octane;Pentane,2,2,4-trimethyl-;
- PSA 0.00000
- LogP 3.07860
Synthetic route

| Conditions | Yield |
|---|---|
| With tin hydride resin; 2,2'-azobis(isobutyronitrile) In benzene at 80℃; for 47h; deamination of other tertiary and secondary amines and dehydroxylation of secondary alcohols; | 89% |

| Conditions | Yield |
|---|---|
| With 2,2-azobisbutyronitrile; tri-n-butyl-tin hydride In benzene for 2h; Heating; | 75% |
| With tri-n-butyl-tin hydride; 2,2'-azobis(isobutyronitrile) In various solvent(s) at 80℃; for 1h; Product distribution; denitrohydrogenation of tertiary nitroalkanes with various methods; | 75% |
-
-
3208-43-3, 4527-76-8
4-tetrahydrofuran-2-yl-butan-2-ol

-
A
-
1004-29-1
2-butanyltetrahydrofuran

-
B
-
3857-17-8, 113611-56-6
2-propyltetrahydropyran

-
C
-
111-65-9
octane

-
D
-
540-84-1
2,2,4-trimethylpentane

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 175℃; under 18751.9 Torr; | A n/a B n/a C 41% D n/a |


-
-
14542-93-9
1,1,3,3-tetramethylbutane isonitrile

-
A
-
107-39-1
2,4,4-trimethyl-1-pentene

-
B
-
540-84-1
2,2,4-trimethylpentane

-
C
-
1088162-71-3
meso-1,1,4,4-tetrakis[bis(trimethylsilyl)methyl]-2,3-dicyano-l,4-diisopropyltetrasilane

-
D
-
1357062-57-7
(E)-1,1,4,4-tetrakis[bis(trimethylsilyl)methyl]-2,3-dicyano-1,4-diisopropyltetrasila-2-ene

| Conditions | Yield |
|---|---|
| In (2)H8-toluene at 20℃; for 5h; | A 68 %Spectr. B 29 %Spectr. C 20 %Spectr. D 24% E 16 %Spectr. |

-
-
80201-75-8
trans-azocyclopropane

-
A
-
594-82-1
tetramethyl-2,2,3,3 butane

-
B
-
540-84-1
2,2,4-trimethylpentane

-
C
-
74-84-0
ethane

-
D
-
74-85-1
ethene

-
E
-
75-19-4
cyclopropane

-
F
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| In various solvent(s) at 45 - 50℃; under 700 Torr; for 8h; Product distribution; Mechanism; Quantum yield; Irradiation; var. wavelengts, other solvents; | A 4.6% B 1% C 2.1% D 11% E 9.2% F 12.5% |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
560-21-4
2,3,3-Trimethyl-pentane

-
B
-
565-75-3
2,3,4-trimethylpentane

-
C
-
540-84-1
2,2,4-trimethylpentane

-
D
-
78-78-4
methylbutane

-
E
-
107-83-5
2-Methylpentane

-
F
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Product distribution; | A n/a B 3.6% C 9.4% D 5.2% E n/a F n/a |

-
-
75-28-5
Isobutane

-
-
74-85-1
ethene

-
A
-
540-84-1
2,2,4-trimethylpentane

-
B
-
78-78-4
methylbutane

-
C
-
107-83-5
2-Methylpentane

-
D
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A 9.4% B 5.2% C n/a D n/a |
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given. Yields of byproduct given; | A 9.4% B 5.2% C n/a D n/a |
| aluminium trichloride-diethyl ether (1/1) at 30℃; under 1520 Torr; for 0.5h; Further byproducts given; | A 7.9% B 3.8% C n/a D n/a |


| Conditions | Yield |
|---|---|
| With hydrogen fluoride at 20 - 25℃; | |
| With hydrogenchloride; aluminium trichloride at -35℃; | |
| durch katalytische Alkylierung; als Ausgangsmaterial dienen die Butan-Buten-Fraktionen der Crackgase oder Crackbenzine; | |
| durch katalytische Alkylierung; als Ausgangsmaterial dienen die Butan-Buten-Fraktionen der Crackgase oder Crackbenzine; | |
| With hydrogen fluoride at 20 - 25℃; |


| Conditions | Yield |
|---|---|
| With hydrogen fluoride at 20 - 25℃; | |
| With hydrogenchloride; aluminium trichloride at -35℃; | |
| With hydrogen fluoride at 20 - 25℃; |

| Conditions | Yield |
|---|---|
| With nickel(II) sulfide; tungsten trisulfide at 215℃; under 183877 Torr; Hydrogenation; | |
| With methanol; copper oxide-chromium oxide barium oxide at 300℃; under 86054.4 Torr; | |
| With platinum Hydrogenation.unter Verwendung von durch elektrische Entladungen aktiviertem Wasserstoff; |

| Conditions | Yield |
|---|---|
| With 2-propylfluoride; hydrogen fluoride at 40℃; |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminium trichloride at -35℃; | |
| With hydrogenchloride; AlCl3 monomethanolate at 28℃; | |
| With hydrogen fluoride at 20 - 25℃; |

| Conditions | Yield |
|---|---|
| With platinum on activated charcoal Hydrogenation; | |
| With sodium tetrahydroborate; Octanoic acid; boron trifluoride diethyl etherate 1.) triglyme, 1 h, RT; 2.) triglyme, 210 deg C, 1 h; Yield given. Multistep reaction; | |
| With hydrogen; platinum on silica at 50 - 250℃; gas chromatograph - hydrogenation microreactor - mass spectrometer; |

| Conditions | Yield |
|---|---|
| durch katalytiche Alkylierung; | |
| With 2-propylfluoride; boron trifluoride at -80 - 25℃; | |
| With hydrogen fluoride; cyclopropane at 40℃; under 5884.06 Torr; |

| Conditions | Yield |
|---|---|
| With BF3*H4O7P2 |

| Conditions | Yield |
|---|---|
| durch katalytische Polymerisation und folgende Hydrierung; |

| Conditions | Yield |
|---|---|
| Hydrogenation; |

| Conditions | Yield |
|---|---|
| With aluminum oxide; silica gel at 110℃; unter Druck und Hydrierung des Reaktionsprodukts an Nickel bei 100grad; |

| Conditions | Yield |
|---|---|
| With hydrogen fluoride at -11℃; | |
| With hydrogen fluoride | |
| With sulfuric acid |

| Conditions | Yield |
|---|---|
| With nickel(II) oxide; iron; magnesium chloride at 250 - 300℃; under 58840.6 Torr; Hydrogenation.Reagens 4: ZnCl2; | |
| With nickel(II) oxide; iron; magnesium chloride at 250 - 300℃; under 58840.6 Torr; Hydrogenation.Reagens 4: AlCl3; | |
| With nickel(II) oxide; iron; magnesium chloride at 250 - 300℃; under 58840.6 Torr; Hydrogenation.Reagens 4: H3PO4-Kieselgur; | |
| Stage #1: isobutene In water at 200℃; under 123762 Torr; Autoclave; Stage #2: With water; iron; nickel at 250℃; under 30003 Torr; for 48h; Temperature; Pressure; Autoclave; |
-
-
187737-37-7
propene

-
-
75-28-5
Isobutane

-
A
-
108-08-7
2,4-dimethylpentane

-
B
-
540-84-1
2,2,4-trimethylpentane

-
C
-
565-59-3
2,3-dimethyl pentane

-
D
-
78-78-4
methylbutane

-
E
-
107-83-5
2-Methylpentane

-
F
-
79-29-8
2,3-dimethylbutane

| Conditions | Yield |
|---|---|
| With zeolite YCe at 70 - 80℃; under 7600 Torr; for 6h; Mechanism; |

-
-
624-64-6
trans-2-Butene

-
-
75-28-5
Isobutane

-
A
-
560-21-4
2,3,3-Trimethyl-pentane

-
B
-
564-02-3
2,2,3-trimethylpentane

-
C
-
565-75-3
2,3,4-trimethylpentane

-
D
-
540-84-1
2,2,4-trimethylpentane

-
E
-
592-13-2
2,5-dimethylhexane

-
F
-
589-43-5
2,4-dimethylhexane

| Conditions | Yield |
|---|---|
| With ultrastable Y zeolite with unit cell size 2.450 nm (USY-1); silica gel at 50℃; for 0.0166667h; Product distribution; other ultrastable Y zeolites (USY) with var. unit sell sizes; the effect of the strength distribution of Broensted acid sites, the concentration of reactants in the pores, and the extent of hydrogen transfer reactions; |

-
-
75-28-5
Isobutane

-
A
-
108-08-7
2,4-dimethylpentane

-
B
-
594-82-1
tetramethyl-2,2,3,3 butane

-
C
-
540-84-1
2,2,4-trimethylpentane

-
D
-
592-13-2
2,5-dimethylhexane

-
E
-
464-06-2
triptane

-
F
-
762-62-9
4,4-dimethylpent-1-ene

| Conditions | Yield |
|---|---|
| at -78.1℃; Product distribution; Irradiation; gamma radiolysis at different irradiation doses, liquid and solid state; |


| Conditions | Yield |
|---|---|
| With Wilkinson's catalyst; diphenyl-phosphinic acid In tetrahydrofuran-d8 at 25℃; for 46h; | 97 % Spectr. |
-
-
5171-85-7
2,2,4,4,5,5,7,7-octamethyloctane

-
A
-
107-39-1
2,4,4-trimethyl-1-pentene

-
B
-
540-84-1
2,2,4-trimethylpentane

-
C
-
107-40-4
2,4,4-trimethylpent-2-ene

| Conditions | Yield |
|---|---|
| In tetralin at 279.1℃; Product distribution; Thermodynamic data; Rate constant; thermolysis, ΔG(excit.), ΔH(excit.), ΔS(excit.); |

-
-
39198-34-0
N,N'-bis(1,1,3,3-tetramethylbutyl)diazene

-
-
540-84-1
2,2,4-trimethylpentane

| Conditions | Yield |
|---|---|
| In benzene at 200℃; for 0.25h; | 85 % Chromat. |
| In benzene at 200℃; for 0.25h; Mechanism; Thermodynamic data; ΔG(excit.), ΔH(excit.), ΔS(excit.); the solvent containts 20 percent thiophenol; | 85 % Chromat. |
-
-
24642-79-3
1,1,3,3-tetramethylcyclobutane

-
-
540-84-1
2,2,4-trimethylpentane

| Conditions | Yield |
|---|---|
| With hydrogen; molybdenum at 191.9℃; Kinetics; Product distribution; |
-
-
24642-79-3
1,1,3,3-tetramethylcyclobutane

-
A
-
540-84-1
2,2,4-trimethylpentane

-
B
-
34557-54-5
methane

-
C
-
74-84-0
ethane

-
D
-
74-98-6
propane

-
E
-
4516-69-2
1,1,3-trimethylcyclopentane

-
F
-
115-11-7
isobutene

| Conditions | Yield |
|---|---|
| With hydrogen; palladium at 201.9℃; Kinetics; Product distribution; on sintered platinum-metal films; | A 73 % Chromat. B n/a C n/a D n/a E 3 % Chromat. F n/a |
| With hydrogen; platinum at 173.9℃; Kinetics; Product distribution; Mechanism; on sintered platinum-metal films; | A 46 % Chromat. B n/a C n/a D n/a E 19 % Chromat. F n/a |
| With hydrogen; platinized copper at 281.9℃; Kinetics; Product distribution; on sintered platinum-metal films; | A n/a B n/a C n/a D n/a E 38 % Chromat. F n/a |

-
-
14542-93-9
1,1,3,3-tetramethylbutane isonitrile

-
-
540-84-1
2,2,4-trimethylpentane

| Conditions | Yield |
|---|---|
| With 2,2'-azobis(isobutyronitrile); tris-(trimethylsilyl)silane In toluene Heating; | 85 % Chromat. |
| With 2,2'-azobis(isobutyronitrile); 1,1,1,2,3,3,3-heptamethyltrisilane In toluene at 348 - 363℃; | 75 % Chromat. |
-
-
145729-01-7
2,3-bis(tert-butylazo)-2,3-dimethylbutane

-
A
-
107-39-1
2,4,4-trimethyl-1-pentene

-
B
-
563-79-1
2,3-Dimethyl-2-butene

-
C
-
594-82-1
tetramethyl-2,2,3,3 butane

-
D
-
540-84-1
2,2,4-trimethylpentane

-
E
-
5846-39-9
2,3,3,4,4-pentamethyl-1-pentene

-
F
-
145729-05-1
2,3-dimethyl-2-(tert-butylazo)butane

| Conditions | Yield |
|---|---|
| In benzene-d6 at 161℃; Rate constant; Product distribution; Kinetics; energy data: ΔH(excit.), ΔS(excit.), ΔG(excit.); var. temperatures, other solvents, also in the presence of thiophenol and irradiation; |

-
-
540-84-1
2,2,4-trimethylpentane

-
-
62574-65-6
2-bromo-2,4,4-trimethylpentane

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; bromine at 10℃; for 1.5h; | 100% |
| With bromine In water at 20℃; for 0.0316667h; Flow reactor; UV-irradiation; | 58 %Chromat. |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride In dichloromethane; water | 89.6% |


| Conditions | Yield |
|---|---|
| With air; 11 wtpercent nickel nanoparticles supported on silica at 750℃; under 760.051 Torr; for 12h; Reagent/catalyst; | 84% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
35426-97-2
2-azido-2,4,4-trimethylpentane

| Conditions | Yield |
|---|---|
| With Perbenzoic acid; 1-azido-1λ3-benzo[d][1,2]iodaoxol-3(1H)-one; 1,1'-azobis(1-cyanocyclohexanenitrile) In 1,2-dichloro-ethane for 3h; Heating; | 76% |

| Conditions | Yield |
|---|---|
| With chromium(VI) oxide; tetrabutylammonium periodite In dichloromethane; acetonitrile at -40℃; for 0.166667h; | 68% |
| With (TPP)FeF; tetrabutyl ammonium fluoride; tetrabutylammonium perchlorate In dichloromethane for 1h; electrolysis: platinum-basket working eleectrode, silver wires counter electrode, 10 mA; | |
| With dihydrogen peroxide; oxalic acid; triphenylphosphine; [Mn2(1,4,7-trimethyl-1,4,7-triazacyclononane)O3][PF6]2 In acetonitrile Product distribution; Further Variations:; Solvents; |

| Conditions | Yield |
|---|---|
| With uranyl nirate hexahydrate In acetone at 20℃; for 24h; Irradiation; | 68% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
17200-53-2
α-(bromomethyl)acrylonitrile

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; potassium carbonate at 130℃; for 8h; Sealed tube; Inert atmosphere; | 64% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
110426-92-1
[(3-bromoprop-1-en-2-yl)sulfonyl]benzene

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; potassium carbonate at 130℃; for 8h; Sealed tube; Inert atmosphere; | 57% |

| Conditions | Yield |
|---|---|
| With aluminium trichloride; tertiary butyl chloride for 4h; Product distribution; Ambient temperature; | 55% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
17435-72-2
ethyl 2-bromomethyl-2-propenoate

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; potassium carbonate at 130℃; for 8h; Time; Sealed tube; Inert atmosphere; | A 55% B 5% |


| Conditions | Yield |
|---|---|
| With hydroxide; nitrogen(II) oxide at 24.85℃; under 740 Torr; Kinetics; | A 26% B 54% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
71-43-2
benzene

-
A
-
253185-03-4, 253185-04-5
tert-butylbenzene

-
B
-
1012-72-2
1,4-di-tert-butylbenzene

-
C
-
1014-60-4
1,3-di-tert-butylbenzene

| Conditions | Yield |
|---|---|
| trifluorormethanesulfonic acid; antimony pentafluoride at 23℃; for 2h; Product distribution; ultra sound; effect of use of var. cat., var. temp., and var. times; | A 49% B n/a C n/a |


| Conditions | Yield |
|---|---|
| With [IPr2*NN]Cu(η2-C6H6) In fluorobenzene at 20℃; Inert atmosphere; Glovebox; Sealed tube; | A 49% B n/a |

-
-
540-84-1
2,2,4-trimethylpentane

-
-
73183-34-3
bis(pinacol)diborane

-
-
1643393-54-7
4,4,5,5-tetramethyl-2-(2,4,4-trimethylpentyl)-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With (1,5-cyclooctadiene)(methoxy)iridium(I) dimer; 3,4,7,8-Tetramethyl-o-phenanthrolin; potassium tert-butylate at 110℃; for 20h; Reagent/catalyst; Glovebox; Inert atmosphere; Sealed tube; | 42% |
-
-
540-84-1
2,2,4-trimethylpentane

-
-
108-24-7
acetic anhydride

-
A
-
233765-95-2
2,4,4-Trimethyl-3-nitro-2-pentanol nitrate

| Conditions | Yield |
|---|---|
| With nitric acid at 14 - 16℃; for 2h; Oxidation; | A 40% B 20% |


| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; potassium carbonate at 130℃; for 8h; Sealed tube; Inert atmosphere; | 37% |

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; (5,10,15,20-tetrakis(p-methoxyphenyl)-21H,23H-porphyrinate)cobalt(II) In benzene at 85℃; | 20% |

| Conditions | Yield |
|---|---|
| With pentanonitrile; Selectfluor; copper(ll) bromide In nitromethane at 60℃; for 4h; Inert atmosphere; | 17% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; aluminium trichloride | |
| With hydrogenchloride; aluminium trichloride |
2,2,4-Trimethylpentane Consensus Reports
2,2,4-Trimethylpentane Standards and Recommendations
2,2,4-Trimethylpentane Specification
1. Introduction of 2,2,4-Trimethylpentane
The 2,2,4-Trimethylpentane, also known as Isooctane, is an organic compound with the formula C8H18. It belongs to the product categories of Industrial/Fine Chemicals; Organics; Analytical Chemistry; HPLC Solvents; Solvents for HPLC & Spectrophotometry; Solvents for Spectrophotometry; Leda Hplc. Its EINECS registry number is 208-759-1. With the CAS registry number 540-84-1, its IUPAC name is 2,2,4-trimethylpentane.
2. Properties of 2,2,4-Trimethylpentane
Physical properties of 2,2,4-Trimethylpentane: (1)ACD/LogP: 4.46; (2)ACD/LogD (pH 5.5): 4.46; (3)ACD/LogD (pH 7.4): 4.46; (4)ACD/BCF (pH 5.5): 1435.33; (5)ACD/BCF (pH 7.4): 1435.33; (6)ACD/KOC (pH 5.5): 6329.15; (7)ACD/KOC (pH 7.4): 6329.15; (8)#Freely Rotating Bonds: 2; (9)Index of Refraction: 1.4; (10)Molar Refractivity: 39.03 cm3; (11)Molar Volume: 161 cm3; (12)Surface Tension: 20.5 dyne/cm; (13)Density: 0.709 g/cm3; (14)Enthalpy of Vaporization: 30.79 kJ/mol; (15)Boiling Point: 98.8 °C at 760 mmHg; (16)Vapour Pressure: 45.2 mmHg at 25°C.
3. Structure Descriptors of 2,2,4-Trimethylpentane
(1)Canonical SMILES: CC(C)CC(C)(C)C
(2)InChI: InChI=1S/C8H18/c1-7(2)6-8(3,4)5/h7H,6H2,1-5H3
(3)InChIKey: NHTMVDHEPJAVLT-UHFFFAOYSA-N
4. Preparation of 2,2,4-Trimethylpentane
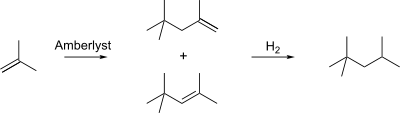
2,2,4-Trimethylpentane is synthesized industrially from isobutylene. Isobutylene is first dimerized using an Amberlyst catalyst to produce a mixture of iso-octenes. Hydrogenation of this mixture produces 2,2,4-trimethylpentane.
5. Use of 2,2,4-Trimethylpentane
2,2,4-Trimethylpentane can be used to produce 1-chloro-2,2,4-trimethyl-pentane at temperature of 85 °C. This reaction will need reagent sulfuryl chloride and solvent benzene. The yield is about 20%.

6. Health Hazard Information of 2,2,4-Trimethylpentane
Acute Effects:
During an accident, 2,2,4-trimethylpentane penetrated the skin of a human which caused necrosis of the skin and tissue in the hand and required surgery. No other information is available on the acute effects in humans.
Irritation of the lungs, edema, and hemorrhage have been reported in rodents acutely exposed by inhalation and injection.
Central nervous system (CNS) depression has been reported in mice following acute inhalation exposure.
Chronic Effects (Noncancer):
No information is available on the chronic effects of 2,2,4-trimethylpentane in humans.
Kidney and liver effects have been observed in rats chronically exposed via gavage and inhalation.
EPA has not established a Reference Concentration (RfC) or a Reference Dose (RfD) for 2,2,4-trimethylpentane.
Reproductive/Developmental Effects:
No information is available on the reproductive or developmental effects of 2,2,4-trimethylpentane in humans or animals.
Cancer Risk:
No information is available on the carcinogenic effects of 2,2,4-trimethylpentane in humans or animals.
EPA has not classified 2,2,4-trimethylpentane with respect to potential carcinogenicity.
7. Other details of 2,2,4-Trimethylpentane
When you are using this chemical, please be cautious about it as the following:
2,2,4-Trimethylpentane may catch fire in contact with air and only need brief contact with an ignition source which has a very low flash point or evolve highly flammable gases in contact with water. It may cause damage to health. In addition, it is irritating to skin. This chemical is very toxic to aquatic organisms which may cause long-term adverse effects in the aquatic environment. You should keep it away from sources of ignition - No smoking. What's more, you should take precautionary measures against static discharges.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 5408-52-6
- 54087-03-5
- 54087-32-0
- 5408-74-2
- 540-88-5
- 54088-65-2
- 5408-86-6
- 54090-08-3
- 54090-41-4
- 54092-09-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View