-
Name
2,4,6-Trichlorobenzoic acid
- EINECS
- CAS No. 50-43-1
- Article Data17
- CAS DataBase
- Density 1.635 g/cm3
- Solubility
- Melting Point 160-164 °C(lit.)
- Formula C7H3Cl3O2
- Boiling Point 329.7 °C at 760 mmHg
- Molecular Weight 225.459
- Flash Point 153.2 °C
- Transport Information
- Appearance beige to brown crystalline powder
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2,4,6-Trichlorobenzoicacid;
- PSA 37.30000
- LogP 3.34500
Synthetic route

| Conditions | Yield |
|---|---|
| With water; triethylamine In acetone at 20℃; for 18h; | 99% |
| With water In acetone at 55℃; Kinetics; Mechanism; Concentration; |
-
-
6324-50-1
1,3,5-trichloro-2-iodobenzene

-
-
124-38-9
carbon dioxide

-
A
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,5-trichloro-2-iodobenzene With n-butyllithium; (tert-butyldimethylsilyl)-tert-butylamine In tetrahydrofuran at -75℃; Stage #2: carbon dioxide In tetrahydrofuran at -75℃; | A 3.2% B 31% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride at 200℃; im Druckrohr; | |
| With sulfuric acid; water; acetic acid |
-
-
23749-65-7
2,4,6-trichlorotoluene

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With nitric acid |

| Conditions | Yield |
|---|---|
| With n-butyllithium 1.) THF, hexane, -50 deg C, 1.5 h, 2.) Et2O, from -50 deg C to RT; Yield given. Multistep reaction; | |
| With methyllithium 1) THF, -30 deg C to -78 deg C; 2) 3 h; Yield given. Multistep reaction; |
-
-
14379-95-4
α,α,α,2,4,6-hexachlorotoluene

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid; Methamphetamin 1) 24 h, r.t.; Yield given. Multistep reaction; |
-
-
24473-01-6
3,3-Dimethyl-1-(2,4,6-trichloro-phenoxy)-butan-2-one

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| at 285 - 305℃; |

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
7647-01-0
hydrogenchloride

-
-
6575-05-9
2,4,6-Trichlorobenzonitrile

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
7664-93-9
sulfuric acid

-
-
23400-04-6
2,4,6-trichloro-benzoic acid amide

-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
854651-12-0
2,2-dibromo-1,3-bis-(2,4,6-trichloro-phenyl)-propane-1,3-dione

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: 2,2,6,6-tetramethylpiperidine; BuLi / tetrahydrofuran; hexane / 2 h / -75 °C 1.2: 91 percent / I2 / tetrahydrofuran; hexane / -75 °C 2.1: BuLi; N-tert-butyl-N-(tert-butyldimethylsilyl)amine / tetrahydrofuran / -75 °C 2.2: 3.2 percent / tetrahydrofuran / -75 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 98 percent / 1a) AlCl3, 1b) AlCl3 / 1a) 4 h, r.t., 1b) 4 h, -10 deg C 2: 76.5 percent / AlCl3 / CS2 / 6 h / Ambient temperature 3: 1) 30percent Oleum, 2) ice / 1) 24 h, r.t. View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 76.5 percent / AlCl3 / CS2 / 6 h / Ambient temperature 2: 1) 30percent Oleum, 2) ice / 1) 24 h, r.t. View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: CHCl3; chlorine 2: hydrochloric acid / Behandlung der Diazoniumchloridloesung mit Kaliumkupfercyanuerloesung 3: concentrated hydrochloric acid / 200 °C / im Druckrohr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrochloric acid / Behandlung der Diazoniumchloridloesung mit Kaliumkupfercyanuerloesung 2: concentrated hydrochloric acid / 200 °C / im Druckrohr View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: hydrochloric acid; glacial acetic acid 2: sulfuric acid 4: HNO3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: HNO3 View Scheme |
-
-
60093-98-3
acetic acid-(2,4,6-trichloro-3-methyl-anilide)

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: sulfuric acid 3: HNO3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: K2CO3 / acetone 2: 285 - 305 °C View Scheme |

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| In ethanol; dichloromethane at 25℃; Kinetics; |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
217479-60-2
(2,4,6-trichlorophenyl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-trichlorobenzoic acid With borane-dimethyl sulfide complex In tetrahydrofuran at 22℃; Inert atmosphere; Cooling with ice; Reflux; Stage #2: With methanol In tetrahydrofuran | 100% |
| With aluminium(III) triflate; tris(2,4-pentanedionato)ruthenium(III); hydrogen; [2-((diphenylphospino)methyl)-2-methyl-1,3-propanediyl]bis[diphenylphosphine] In methanol; water; toluene at 160℃; under 45004.5 Torr; for 24h; Autoclave; | 67 %Chromat. |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
86569-78-0
methyl 2,4,6-trichlorobenzoate

| Conditions | Yield |
|---|---|
| In diethyl ether | 94% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; | 86% |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
16463-22-2
1,3,5-trichloro[2-2H]benzene

| Conditions | Yield |
|---|---|
| With water-d2; silver(I) acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 120℃; for 16h; Inert atmosphere; regioselective reaction; | 71% |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
594-31-0, 34716-91-1, 20265-29-6
triphenylantimony dichloride

| Conditions | Yield |
|---|---|
| With CH3ONa In toluene 2,4,6-trichlorobenzoic acid and CH3ONa added to soln. of Ph3SbCl2 in toluene under stirring (molar ratio=1:1), mixt. refluxed for 8 h; soln. filtered, filtrate gradually evapd. under vac., recrystn. from petroleum ether/CH2Cl2 (1/1); elem. anal.; | 68% |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
594-31-0, 34716-91-1, 20265-29-6
triphenylantimony dichloride

| Conditions | Yield |
|---|---|
| With CH3ONa In toluene 2,4,6-trichlorobenzoic acid and CH3ONa added to soln. of Ph3SbCl2 in toluene under stirring (molar ratio=2:1), mixt. refluxed for 8 h; soln. filtered, filtrate gradually evapd. under vac., recrystn. from petroleum ether/CH2Cl2 (1/1); elem. anal.; | 65% |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
16894-69-2
tetraphenylantimony(V) bromide

| Conditions | Yield |
|---|---|
| With CH3ONa In toluene 2,4,6-trichlorobenzoic acid and CH3ONa added to soln. of Ph4SbBr in toluene under stirring (molar ratio=1:1), mixt. refluxed for 8 h; soln. filtered, filtrate gradually evapd. under vac., recrystn. from petroleum ether/CH2Cl2 (1/1); elem. anal.; | 61% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 288h; | 60.13% |

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; silver carbonate In 1,4-dioxane; dimethyl sulfoxide at 120℃; for 12h; Sealed tube; diastereoselective reaction; | 40% |
-
-
504-24-5
4-aminopyridine

-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
1258291-82-5
2,4,6-trichloro-N-(pyridin-4-yl)benzamide

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; HATU In N,N-dimethyl-formamide at 20℃; Inert atmosphere; | 24% |
-
-
36997-05-4
2-methoxyacrylic acid ethyl ester

-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
A
-
108-70-3
1,3,5-trichlorobenzene

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; silver carbonate In 1,4-dioxane; dimethyl sulfoxide at 130℃; for 24h; Solvent; Sealed tube; Inert atmosphere; stereoselective reaction; | A n/a B 24% C 16% D n/a |

-
-
36997-05-4
2-methoxyacrylic acid ethyl ester

-
-
50-43-1
2,4,6-trichlorobenzoic acid

| Conditions | Yield |
|---|---|
| With palladium(II) trifluoroacetate; silver carbonate In N,N-dimethyl-formamide at 130℃; for 24h; Sealed tube; Inert atmosphere; stereoselective reaction; | 20% |
-
-
50-43-1
2,4,6-trichlorobenzoic acid

-
-
14861-17-7
2,4-dichloro-4'-aminodiphenyl ether

| Conditions | Yield |
|---|---|
| Stage #1: 2,4,6-trichlorobenzoic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 1h; Stage #2: 2,4-dichloro-4'-aminodiphenyl ether In N,N-dimethyl-formamide | 13% |

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride | |
| With phosphorus pentachloride | |
| With thionyl chloride |
2,4,6-Trichlorobenzoic acid Chemical Properties
Molecule structure of 2,4,6-Trichlorobenzoic acid (50-43-1):
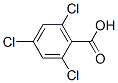
IUPAC Name: 2,4,6-Trichlorobenzoic acid
Molecular Weight: 225.45652 g/mol
Molecular Formula: C7H3Cl3O2
Density: 1.635 g/cm3
Melting Point: 160-164 °C(lit.)
Boiling Point: 329.7 °C at 760 mmHg
Flash Point: 153.2 °C
Index of Refraction: 1.611
Molar Refractivity: 47.86 cm3
Molar Volume: 137.8 cm3
Polarizability: 18.97×10-24 cm3
Surface Tension: 56 dyne/cm
Enthalpy of Vaporization: 60.4 kJ/mol
Vapour Pressure: 7.02E-05 mmHg at 25 °C
XLogP3: 2.9
H-Bond Donor: 1
H-Bond Acceptor: 2
Rotatable Bond Count: 1
Exact Mass: 223.919862
MonoIsotopic Mass: 223.919862
Topological Polar Surface Area: 37.3
Heavy Atom Count: 12
Complexity: 174
Canonical SMILES: C1=C(C=C(C(=C1Cl)C(=O)O)Cl)Cl
InChI: InChI=1S/C7H3Cl3O2/c8-3-1-4(9)6(7(11)12)5(10)2-3/h1-2H,(H,11,12)
InChIKey: RAFFVQBMVYYTQS-UHFFFAOYSA-N
Product Categories: FINE Chemical & INTERMEDIATES;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Organic acids;C7;Carbonyl Compounds;Carboxylic Acids
2,4,6-Trichlorobenzoic acid Uses
2,4,6-Trichlorobenzoic acid (50-43-1) is used for the synthesis of medicine, insecticides and fungicides.
2,4,6-Trichlorobenzoic acid Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | subcutaneous | 1200mg/kg (1200mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: MUSCLE WEAKNESS LUNGS, THORAX, OR RESPIRATION: DYSPNEA | Biochemical Pharmacology. Vol. 13, Pg. 1538, 1964. |
2,4,6-Trichlorobenzoic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-37/39
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S37/39:Wear suitable gloves and eye/face protection.
WGK Germany: 3
RTECS: DH8050000
Hazard Note: Irritant
2,4,6-Trichlorobenzoic acid Specification
2,4,6-Trichlorobenzoic acid (50-43-1) is also named as 4-09-00-01010 (Beilstein Handbook Reference) ; AI3-33271 ; BRN 1874127 ; Benzoic acid, 2,4,6-trichloro- . 2,4,6-Trichlorobenzoic acid (50-43-1) is beige to light brown crystalline powder.
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 50432-68-3
- 50432-79-6
- 50433-06-2
- 504-33-6
- 50434-36-1
- 50436-33-4
- 5043-81-2
- 50439-35-5
- 50439-37-7
- 50439-45-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View