-
Name
2,4-Diamino-6-hydroxypyrimidine
- EINECS 200-254-4
- CAS No. 56-06-4
- Article Data16
- CAS DataBase
- Density 1.84 g/cm3
- Solubility
- Melting Point 285-286 °C (dec.)(lit.)
- Formula C4H6N4O
- Boiling Point 288.5 °C at 760 mmHg
- Molecular Weight 126.118
- Flash Point 128.3 °C
- Transport Information
- Appearance white solid
- Safety 22-24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 4(1H)-Pyrimidinone,2,6-diamino- (8CI,9CI);2,4-Diamino-6-hydroxypyrimidine;2,4-Diamino-6-pyrimidinone;2,6-Diamino-3,4-dihydropyrimidin-4-one;2,6-Diamino-4(1H)-pyrimidinone;2,6-Diamino-4(3H)-pyrimidinone;2,6-Diamino-4-hydroxypyrimidine;2,6-Diamino-4-pyrimidinol;2,6-Diaminopyrimidin-4-one;2,6-Diaminopyrimidine-4(3H)-one;6-Aminoisocytosine;6-Hydroxy-2,4-pyrimidinediamine;NSC 44914;NSC 680818;NSC9302;
- PSA 98.05000
- LogP 0.50900
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: guanidine nitrate; ethyl 2-cyanoacetate With sodium methylate In methanol for 4h; Reflux; Stage #2: With hydrogenchloride In water pH=9; pH-value; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 4h; Reflux; | 66% |
-
-
88075-70-1
2,4-diamino-5-formyl-6-oxo(1H)-pyrimidine

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

| Conditions | Yield |
|---|---|
| With sulfuric acid In methanol for 72h; Ambient temperature; |
-
-
108-98-5
thiophenol

-
-
100061-59-4
NU6038

-
A
-
831-91-4
Benzyl phenyl sulfide

-
B
-
56-06-4
2,6-diaminopyrimidin-4-ol

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 60℃; Rate constant; |


| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 20℃; Rate constant; |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
51953-18-5
pyrimidine-4(3H)-one

-
B
-
156-81-0
2,4-diaminopyrimidine

-
C
-
120-89-8
parabanic acid

-
D
-
108-53-2
isocytosine

-
E
-
71-30-7
Cytosine

-
F
-
144-62-7
oxalic acid

-
G
-
113-00-8
guanidine nitrate

-
H
-
127-17-3
2-oxo-propionic acid

-
I
-
66-22-8
uracil

-
J
-
56-06-4
2,6-diaminopyrimidin-4-ol


-
L
-
57-13-6
urea

-
M
-
56-40-6
glycine

| Conditions | Yield |
|---|---|
| With copper(II) choride dihydrate In water at 80℃; for 24h; pH=7.57; | A 5.6 mg B 0.3 mg C 0.59 mg D 5 mg E 0.13 mg F 0.1 mg G 1.6 mg H 0.0018 mg I 3.8 mg J 0.3 mg K 0.01 mg L 1.7 mg M 0.76 mg |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
51953-18-5
pyrimidine-4(3H)-one

-
B
-
156-81-0
2,4-diaminopyrimidine

-
C
-
113-00-8
guanidine nitrate

-
D
-
66-22-8
uracil

-
E
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
F
-
57-13-6
urea

| Conditions | Yield |
|---|---|
| With manganese(II) chloride tetrahydrate In water at 80℃; for 24h; pH=7.57; | A 0.3 mg B 0.02 mg C 0.1 mg D 0.03 mg E 0.06 mg F 0.005 mg |


| Conditions | Yield |
|---|---|
| With sodium methylate |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
51953-18-5
pyrimidine-4(3H)-one

-
B
-
1455-77-2
guanazole

-
C
-
120-89-8
parabanic acid

-
D
-
849585-22-4
LACTIC ACID

-
E
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
F
-
328-42-7
Oxalacetic acid

-
G
-
2491-15-8
formylglycine

-
H
-
110-15-6
succinic acid

-
I
-
71-30-7
Cytosine

-
J
-
120-73-0
purine

-
K
-
144-62-7
oxalic acid

-
L
-
113-00-8
guanidine nitrate

-
M
-
127-17-3
2-oxo-propionic acid

-
N
-
66-22-8
uracil

-
O
-
56-06-4
2,6-diaminopyrimidin-4-ol


-
Q
-
57-13-6
urea

-
R
-
56-40-6
glycine

-
S
-
302-72-7
rac-Ala-OH

-
T
-
18588-61-9
2,4-diamino-pyrimidine-5-carboxylic acid

| Conditions | Yield |
|---|---|
| With ferric sulfate nonahydrate; water at 80℃; for 24h; |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
23147-58-2, 110822-84-9, 110822-85-0
glycolaldehyde dimer

-
B
-
1455-77-2
guanazole

-
C
-
849585-22-4
LACTIC ACID

-
D
-
328-42-7
Oxalacetic acid

-
E
-
110-15-6
succinic acid

-
F
-
120-73-0
purine

-
G
-
144-62-7
oxalic acid

-
H
-
66-22-8
uracil

-
I
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
J
-
57-13-6
urea

| Conditions | Yield |
|---|---|
| With copper(II) chloride tetrahydrate; water at 80℃; for 24h; |

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
A
-
51953-18-5
pyrimidine-4(3H)-one

-
B
-
120-89-8
parabanic acid

-
C
-
73-40-5
2-amino-1,9-dihydro-6H-purin-6-one

-
D
-
328-42-7
Oxalacetic acid

-
E
-
110-15-6
succinic acid

-
F
-
144-62-7
oxalic acid

-
G
-
127-17-3
2-oxo-propionic acid

-
H
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
I
-
57-13-6
urea

-
J
-
18588-61-9
2,4-diamino-pyrimidine-5-carboxylic acid

-
K
-
18514-52-8
2,3-diaminomaleonitrile

| Conditions | Yield |
|---|---|
| With water; manganese(ll) chloride at 80℃; for 24h; |

-
-
141-82-2
malonic acid

-
-
113-00-8
guanidine nitrate

-
-
57-13-6
urea

-
A
-
1004-38-2
2,4,6-triaminopyrimidine

-
B
-
4425-67-6
2-aminopyrimidine-4,6-diol

-
C
-
67-52-7
BARBITURIC ACID

-
D
-
108-78-1
2,4,6-triamino-s-triazine

-
E
-
3546-50-7
pyrimidine-2,4,5-triamine

-
G
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
H
-
31458-45-4
2-hydroxy-4,6-diaminopyrimidine

-
I
-
2345-56-4
Malonamic acid

| Conditions | Yield |
|---|---|
| at 65℃; for 120h; |


| Conditions | Yield |
|---|---|
| With sodium ethanolate In ethanol at 50 - 70℃; under 1500.15 Torr; for 1.5h; Large scale; |
-
-
20099-89-2
4-Cyanophenacyl bromide

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

| Conditions | Yield |
|---|---|
| Stage #1: 2,6-diaminopyrimidin-4-ol With sodium acetate In water at 100℃; for 0.333333h; Stage #2: 4-Cyanophenacyl bromide In methanol at 100℃; | 100% |
| Stage #1: 2,6-diaminopyrimidin-4-ol With sodium acetate In water at 100℃; for 0.333333h; Stage #2: 4-Cyanophenacyl bromide In methanol; water at 100℃; | 100% |
-
-
333-20-0
potassium thioacyanate

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
-
22288-75-1
2,4-diamino-6-hydroxy-5-thiocyanatopyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: potassium thioacyanate; 2,6-diaminopyrimidin-4-ol With acetic acid at 25 - 95℃; Inert atmosphere; Stage #2: With bromine at 10 - 20℃; for 2h; | 100% |

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 5h; | 99% |

| Conditions | Yield |
|---|---|
| With bromine; sodium hydrogencarbonate In methanol; water 2 h stirring, then standing overnight; | 97% |
| With N-Bromosuccinimide; montmorillonite K-10; tetrabutylammomium bromide for 0.0416667h; microwave irradiation; | 92% |

| Conditions | Yield |
|---|---|
| With acetic acid; sodium nitrite In water at 80℃; | 97% |
| With acetic acid; sodium nitrite In water | 97% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid at 30 - 35℃; for 2h; | 96.4% |
| With sodium nitrite In water at 0 - 5℃; under 750.075 Torr; for 0.5h; Large scale; |
-
-
138911-94-1
2-hydroxymethylene-1-tetralone sodium salt

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

| Conditions | Yield |
|---|---|
| With phosphoric acid at 100℃; for 90h; | 95% |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 20℃; pH=7; Electrolysis; Green chemistry; | 95% |
-
-
959934-74-8
3-[(2-methyl-4-oxo-4H-chromen-8-yl)methylene]pentane-2,4-dione

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
-
959934-79-3
8-(6-acetyl-2-amino-4-hydroxy-7-methyl-5,8-dihydropyrido[2,3-d]-pyrimidin-5-yl)-2-methyl-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 48h; Reflux; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.133333h; | 94% |


| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 20℃; pH=7; Electrolysis; Green chemistry; | 93% |

| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.216667h; | 92% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.183333h; | 92% |


-
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
-
857036-63-6
Eu((NH2)2C4N2H2O)2(H2O)3(NO3)2(1+)*NO3(1-)=Eu((NH2)2C4N2H2O)2(H2O)3(NO3)3

| Conditions | Yield |
|---|---|
| In methanol pyrimidine-compound was added to methanol, then hydrated Eu(NO3)3 was added, the soln. was stirred for 24 h; slow evapd.; elem. anal.; | 91% |

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 20℃; pH=7; Electrolysis; Green chemistry; | 91% |

| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.166667h; | 91% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.216667h; | 91% |


-
-
56-06-4
2,6-diaminopyrimidin-4-ol

| Conditions | Yield |
|---|---|
| In methanol pyrimidine-compound was added to methanol, then hydrated TbCl3 was added, the soln. was stirred for 24 h; slow evapd.; elem. anal.; | 90.4% |

| Conditions | Yield |
|---|---|
| With N-benzyl-N,N,N-triethylammonium chloride In water at 90℃; for 6h; | 90% |

| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.3h; | 90% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.2h; | 90% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.2h; | 90% |


| Conditions | Yield |
|---|---|
| In water; ethyl acetate at 50℃; for 24h; | 90% |
-
-
2051-49-2
n-hexanoic anhydride

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
-
1402591-91-6
N,N'-(6-hydroxypyrimidine-2,4-diyl)dihexanamide

| Conditions | Yield |
|---|---|
| for 4h; Reflux; | 89% |

| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.233333h; | 89% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.266667h; | 89% |


| Conditions | Yield |
|---|---|
| With silica-coated iron oxide nanomagnetic particles-bonded S-sulfonic acid In neat (no solvent) at 100℃; for 0.333333h; | 89% |

-
-
56-06-4
2,6-diaminopyrimidin-4-ol

-
-
154597-38-3
(4RS)-4-ethyl-7,8-dihydro-4-hydroxy-7-dimethylaminomethylene-1H-pyrano<3,4-f>indolizine-3,6,10(4H)-trione

| Conditions | Yield |
|---|---|
| In acetic acid for 20h; Heating; | 88% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; potassium fluoride In ethanol at 80℃; for 0.666667h; | 88% |
2,4-Diamino-6-hydroxypyrimidine Specification
The IUPAC name of 2,4-Diamino-6-hydroxypyrimidine is 2,6-diamino-1H-pyrimidin-4-one. With the CAS registry number 56-06-4, it is also named as 4(1H)-Pyrimidinone, 2,6-diamino-. The product's categories are Pyrimidine; APIs & Intermediate; Pyridines, Pyrimidines, Purines and Pteredines; Chemical Amines; 13C & 2H Sugars; Amines; Bases & Related Reagents; Heterocycles; Nucleotides; Carbohydrates & Derivatives, and the other registry numbers are 40306-60-3; 41982-31-4; 863767-89-9. Besides, it is white solid, which should be stored in sealed, dark, cool, ventilated and dry place away from fire and heat source. In addition, it is light sensitive.
The other characteristics of this product can be summarized as: (1)EINECS: 200-254-4; (2)ACD/LogP: -1.66; (3)# of Rule of 5 Violations: 1; (4)ACD/LogD (pH 5.5): -1.66; (5)ACD/LogD (pH 7.4): -1.66; (6)ACD/BCF (pH 5.5): 1; (7)ACD/BCF (pH 7.4): 1; (8)ACD/KOC (pH 5.5): 2.95; (9)ACD/KOC (pH 7.4): 2.98; (10)H bond acceptors: 5; (11)H bond donors: 5; (12)Freely Rotating Bonds: 1; (13)Index of Refraction: 1.798; (14)Molar Refractivity: 29.21 cm3; (15)Molar Volume: 68.4 cm3; (16)Surface Tension: 94.7 dyne/cm; (17)Density: 1.84 g/cm3; (18)Flash Point: 128.3 °C; (19)Melting point: 285-286 °C; (20)Enthalpy of Vaporization: 52.78 kJ/mol; (21)Boiling Point: 288.5 °C at 760 mmHg; (22)Vapour Pressure: 0.00232 mmHg at 25 °C.
Preparation of 2,4-Diamino-6-hydroxypyrimidine: please put Guanidine nitrate in the solution of Sodium methoxide (or 50% NaOH) to heat and stir. After backflow half-hour, please drop Methyl cyanoacetate to reflow 2 hours. After the reaction complete, please heat the reclaimed Methanol. And then put the residues in the hot water to dissolve. When the temperature reach 80 °C, please add acetic acid to adjust pH=8. Then please separate crystallographic product out. After cooling below 20 °C, you would get this chemical by filtration, washing and drying.

Uses of 2,4-Diamino-6-hydroxypyrimidine: it is used for detection of nitrate and nitrite. It is also used in organic synthesis and pharmaceutical intermediates. And it is used for the production of AZM, minoxidilum and olic acid. Furthermore, it can react with 1,3-Dichloro-propan-2-one to get 5-Chloromethyl-furo[2,3-d]pyrimidine-2,4-diamine.
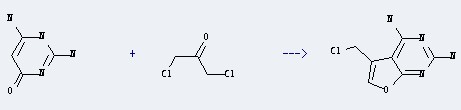
This reaction needs Dimethylformamide at temperature of 20 °C for 24 hours. The yield is 80 %.
When you are using this chemical, please be cautious about it as the following: it is irritating to eyes, respiratory system and skin. Please do not breathe dust. And please avoid contact with skin and eyes. You should wear suitable protective clothing. Moreover, in case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
People can use the following data to convert to the molecule structure.
(1)SMILES:O=C/1/N=C(/N)NC(\N)=C\1
(2)InChI:InChI=1/C4H6N4O/c5-2-1-3(9)8-4(6)7-2/h1H,(H5,5,6,7,8,9)
(3)InChIKey:SWELIMKTDYHAOY-UHFFFAOYAR
(4)Std. InChI:InChI=1S/C4H6N4O/c5-2-1-3(9)8-4(6)7-2/h1H,(H5,5,6,7,8,9)
(5)Std. InChIKey:SWELIMKTDYHAOY-UHFFFAOYSA-N
Related Products
- 20,22-DIHYDRODIGITOXIN
- 20,29,30-Trinorlupane,(17alpha)-
- 20-ETHYL-6-β,8-DIHYDROXY-1-α-METHOXY-4-METHYLHETERATISAN-14-ONE
- 20-Ethylprostaglandin F2-alpha
- 20-Isopropylcholanthrene
- 20-METHYLCHOLANTHREN-15-ONE
- 20-METHYLCHOLANTHRENE PICRATE
- 20-METHYLCHOLANTHRENE-TRINITRO-BENZENE
- 20(S)-Ginsenoside C-K
- 2,10-DIFLUOROBENZO(rst)PENTAPHENE
- 56068-50-9
- 56070-16-7
- 56071-69-3
- 56071-70-6
- 56073-07-5
- 56073-10-0
- 56074-20-5
- 56076-20-1
- 56077-28-2
- 56077-78-2
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View