-
Name
2-Acetamido-5-nitropyridine
- EINECS -0
- CAS No. 5093-64-1
- Article Data20
- CAS DataBase
- Density 1.419 g/cm3
- Solubility
- Melting Point 199-203 °C(lit.)
- Formula C7H7N3O3
- Boiling Point 437.1 °C at 760 mmHg
- Molecular Weight 181.151
- Flash Point 218.1 °C
- Transport Information
- Appearance
- Safety 26-36/37/39
- Risk Codes 37/38-41
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetamide,N-(5-nitro-2-pyridyl)- (8CI);Pyridine, 2-acetamido-5-nitro- (6CI,7CI);2-Acetylamino-5-nitropyridine;N-(5-Nitropyridin-2-yl)acetamide;NSC 402463;
- PSA 87.81000
- LogP 1.54440
Synthetic route
-
-
4214-76-0
5-nitro-pyridin-2-ylamine

-
A
-
29958-14-3
N-(5-aminopyridin-2-yl)acetamide

-
B
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| With sulfuric acid In acetic anhydride | A n/a B 98% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 0 - 20℃; | 86% |
| With pyridine; dmap at 0 - 25℃; for 5h; | 86% |
| at 130℃; for 1.5h; | 81% |

| Conditions | Yield |
|---|---|
| With pyridine In tetrahydrofuran at 0 - 20℃; for 2h; | 80.1% |
| With triethylamine In dichloromethane at 20℃; for 6h; | 77% |
| With dmap; triethylamine In tetrahydrofuran; ethyl acetate | |
| With dmap; triethylamine In tetrahydrofuran for 24h; Heating / reflux; |
-
-
4214-76-0
5-nitro-pyridin-2-ylamine

-
-
127-19-5
N,N-dimethyl acetamide

-
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl acetamide With 1,1'-carbonyldiimidazole at 120 - 125℃; for 0.5h; Inert atmosphere; Stage #2: 5-nitro-pyridin-2-ylamine at 60 - 65℃; for 5.5h; Inert atmosphere; | 70% |
-
-
1122-58-3
dmap

-
-
4214-76-0
5-nitro-pyridin-2-ylamine

-
-
75-36-5
acetyl chloride

-
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| With potassium carbonate; triethylamine In dichloromethane | 49% |
| With potassium carbonate; triethylamine In dichloromethane | 49% |


| Conditions | Yield |
|---|---|
| Stage #1: acetamide With sodium hydride In dimethyl sulfoxide for 2h; Stage #2: 3-nitropyridine With potassium hexacyanoferrate(III) In dimethyl sulfoxide at 20℃; for 2h; | 34% |

| Conditions | Yield |
|---|---|
| With dmap; N2; triethylamine In CH2C12 |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
29958-14-3
N-(5-aminopyridin-2-yl)acetamide

| Conditions | Yield |
|---|---|
| With hydrogen; palladium on activated charcoal In ethanol for 3.5h; | 98% |
| With water; ammonium chloride; zinc In ethanol at 20℃; for 5h; | 84% |
| With hydrogen; palladium on activated charcoal In methanol |

| Conditions | Yield |
|---|---|
| In ethanol; chloroform | 20% |
| In ethanol; chloroform | 20% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether In acetonitrile for 32h; Heating; |
-
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 18-crown-6 / acetonitrile / 32 h / Heating 2: aq. KHSO4; 18-crown-6; sodium chlorodifluoroacetate / acetonitrile / 1 h / Heating View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
104-15-4
toluene-4-sulfonic acid

-
-
1449012-40-1
C7H7N3O3*C7H8O3S

| Conditions | Yield |
|---|---|
| In toluene for 2h; Reflux; |
-
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: zinc; ammonium chloride; water / ethanol / 5 h / 20 °C 2.1: thionyl chloride; triethylamine / dichloromethane / 4 h / 20 °C 2.2: 20 °C View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C 3.1: triethylamine / ethanol / 3 h / pH 9.5 / Reflux View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
1246468-33-6
N-{4-[5-(acetylamino)-1H-pyrrolo[3,2-b]pyridin-2-yl]phenyl}-1-(phenylacetyl)-L-prolinamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C 3.1: triethylamine / ethanol / 3 h / pH 9.5 / Reflux 4.1: polyphosphoric acid / 1.25 h / 90 °C / Inert atmosphere 5.1: hydrogenchloride; water / 2 h / Reflux 6.1: HATU; N-ethyl-N,N-diisopropylamine / acetonitrile / 25 °C 7.1: triethylamine / acetonitrile / 0.6 h / 0 - 25 °C View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
769912-56-3
C7H10N4O

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
1246470-71-2
C17H16N4O2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C 3.1: triethylamine / ethanol / 3 h / pH 9.5 / Reflux 4.1: polyphosphoric acid / 1.25 h / 90 °C / Inert atmosphere View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
1246470-72-3
C13H12N4

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C 3.1: triethylamine / ethanol / 3 h / pH 9.5 / Reflux 4.1: polyphosphoric acid / 1.25 h / 90 °C / Inert atmosphere 5.1: hydrogenchloride; water / 2 h / Reflux View Scheme |
-
-
5093-64-1
2-acetamido-5-nitropyridine

-
-
1246470-73-4
C26H25N5O2

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: hydrogen; palladium 10% on activated carbon / methanol / 6 h / 2585.81 Torr 2.1: sodium nitrite; hydrogenchloride / water / 0.75 h / 0 °C 2.2: 16 h / 0 - 20 °C 3.1: triethylamine / ethanol / 3 h / pH 9.5 / Reflux 4.1: polyphosphoric acid / 1.25 h / 90 °C / Inert atmosphere 5.1: hydrogenchloride; water / 2 h / Reflux 6.1: HATU; N-ethyl-N,N-diisopropylamine / acetonitrile / 25 °C View Scheme |
2-Acetamido-5-nitropyridine Chemical Properties
Product Name: 2-Acetamido-5-nitropyridine (CAS NO.5093-64-1)
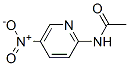
Molecular Formula: C7H7N3O3
Molecular Weight: 181.15g/mol
Mol File: 5093-64-1.mol
Melting Point: 199-203 °C(lit.)
Boiling point: 437.1 °C at 760 mmHg
Flash Point: 1.419 g/cm3
Density: 218.1 °C
Surface Tension: 63.8 dyne/cm
Enthalpy of Vaporization: 69.36 kJ/mol
Vapour Pressure: 7.69E-08 mmHg at 25°C
XLogP3-AA: 0.2
H-Bond Donor: 1
H-Bond Acceptor: 4
Structure Descriptors of 2-Acetamido-5-nitropyridine (CAS NO.5093-64-1):
IUPAC Name: N-(5-nitropyridin-2-yl)acetamide
Canonical SMILES: CC(=O)NC1=NC=C(C=C1)[N+](=O)[O-]
InChI: InChI=1S/C7H7N3O3/c1-5(11)9-7-3-2-6(4-8-7)10(12)13/h2-4H,1H3,(H,8,9,11)
InChIKey: XKAASKOXADTLIG-UHFFFAOYSA-N
Product Categories: Pyridine; C7 and C8; Heterocyclic Building Blocks; Pyridines
2-Acetamido-5-nitropyridine Safety Profile
Safety Information of 2-Acetamido-5-nitropyridine (CAS NO.5093-64-1):
Hazard Codes: Xi 
Risk Statements: 37/38-41
37: Irritating to the respiratory system
38: Irritating to the skin
41: Risk of serious damage to eyes
Safety Statements: 26-36/37/39
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37: Wear suitable gloves
39: Wear eye/face protection
WGK Germany: 3
Hazard Note: Irritant
HazardClass: IRRITANT
2-Acetamido-5-nitropyridine Specification
2-Acetamido-5-nitropyridine , its CAS NO. is 5093-64-1, the synonym is N-{5-nitro-2-pyridinyl}acetamide .
Related Products
- 2-Acetamido-5-nitropyridine
- 5093-70-9
- 50940-49-3
- 5094-33-7
- 5095-80-7
- 509-60-4
- 5096-11-7
- 50965-80-5
- 50966-74-0
- 509-67-1
- 5096-73-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View