-
Name
2-AMINO-1-PROPENE-1,1,3-TRICARBONITRILE
- EINECS 212-777-5
- CAS No. 868-54-2
- Article Data19
- CAS DataBase
- Density 1.262 g/cm3
- Solubility
- Melting Point 171-173 °C (lit. )
- Formula C6H4N4
- Boiling Point 591.9 °C at 760 mmHg
- Molecular Weight 132.125
- Flash Point 311.8 °C
- Transport Information
- Appearance light pink to beige or light brownish
- Safety 22-24/25
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms (1-Amino-2-cyanoethylidene)malononitrile;1,1,3-Tricyano-2-amino-1-propene;1,1,3-Tricyano-2-aminopropene;2-Amino-1,1,3-propenetricarbonitrile;2-Amino-1,1,3-tricyano-1-propene;2-Amino-1,1,3-tricyanopropene;2-Amino-1-propene-1,1,3-tricarbonitrile;Malononitrile, dimer;NSC 77901;TCAP;TRIAP;U 9189;Upjohn 9189;
- PSA 97.39000
- LogP 0.86034
Synthetic route

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 0.5h; Product distribution; Heating; further solvents and reagents; | 87% |
| With potassium hydroxide In ethanol for 0.5h; Heating; | 87% |
| With sodium hydride In dimethyl sulfoxide; paraffin oil at 20℃; | 87% |
-
-
109-77-3
malononitrile

-
A
-
24571-64-0
4,6-Diamino-2-cyanmethyl-3,4-pyridincarbonitril

-
B
-
14203-74-8
1,3,5-triamino-2,4,6-tricyanobenzene

-
C
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| [Cu2(OAc)4(H2O)2] In 1,4-dioxane for 72h; Heating; | A 26% B 7% C 14% |

-
-
109-99-9
tetrahydrofuran

-
-
60-29-7
diethyl ether

-
-
109-77-3
malononitrile

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| Reaktion der Natrium-Verbindung; |


| Conditions | Yield |
|---|---|
| Reaktion der Natrium-Verbindung; |
-
-
7647-01-0
hydrogenchloride

-
-
109-77-3
malononitrile

-
-
71-43-2
benzene

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
64-17-5
ethanol

-
-
10544-72-6
dinitrogen tetraoxide

-
-
109-77-3
malononitrile

-
A
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
B
-
105-56-6
ethyl 2-cyanoacetate

| Conditions | Yield |
|---|---|
| Reaktion der Natrium-Verbindung; |

-
-
109-77-3
malononitrile

-
A
-
2638-14-4
2-cyanomethyl-1,1,3,3-tetracyanopropene

-
B
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In water |
-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
152193-58-3
3,3,3-trifluoroprop-1-ynyl-phosphonic acid diethyl ester

-
-
1478881-72-9
diethyl (2,4-diamino-3,5-dicyano-6-(trifluoromethyl)phenyl)phosphonate

| Conditions | Yield |
|---|---|
| With potassium carbonate In toluene Inert atmosphere; Reflux; | 98% |
| With potassium carbonate In toluene Reflux; | 98% |
-
-
22001-17-8
3-trifluoromethylphenylazide

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With Oksipav AP (cocamidopropyldimethylamine oxides) In water for 2h; Knoevenagel Condensation; | 98% |
-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With triethylamine In propan-1-ol at 98℃; for 1h; Solvent; Reagent/catalyst; | 98% |
| With triethylamine In propan-1-ol at 98℃; for 1h; Catalytic behavior; Reagent/catalyst; Solvent; | 98% |

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
89-25-8
edaravone

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 82℃; for 1h; | 98% |

-
-
70254-43-2, 70254-44-3
4-Hydroxy-2-quinolone

-
-
492-88-6
3-ethoxysalicylaldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With pyridine In ethanol for 2h; Reflux; | 98% |

-
-
16883-69-5
N-phenacylpyridinium bromide

-
-
104-88-1
4-chlorobenzaldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
132252-91-6
C26H18ClN5O

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol Heating; | 97% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

-
-
83986-42-9
3-cyano-2,4-diamino-5-salicylidene-5,6-dihydropyridin-6-one

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.0833333h; Heating; | 96% |
-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
384846-43-9
2-[(5,5-dimethyl-3-oxocyclohexen-1-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiopene-3-carbonitrile

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.0833333h; Reflux; | 96% |

-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
696-63-9
4-Methoxybenzenethiol

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 4h; Temperature; Reflux; | 95% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
89-98-5
2-chloro-benzaldehyde

-
-
475478-10-5
2-[(3-oxo-1-cyclohexen-1-yl)amino]-4,5,6,7-tetrahydro-1-benzo[b]thiophen-3-carbonitrile

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.0833333h; Reflux; | 95% |


| Conditions | Yield |
|---|---|
| With potassium phosphate In methanol at 20℃; for 2h; Michael Addition; | 95% |

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 82℃; for 1h; | 95% |

-
-
70254-43-2, 70254-44-3
4-Hydroxy-2-quinolone

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

| Conditions | Yield |
|---|---|
| With pyridine In ethanol for 2h; Reagent/catalyst; Solvent; Reflux; | 95% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
28317-57-9
phenylazo acetylacetonitrile

-
-
182863-49-6
2-amino-5-cyano-6-dicyanomethino-4-methyl-3-phenylhydrazo-pyridine

| Conditions | Yield |
|---|---|
| With ammonium acetate at 160℃; for 1h; | 94% |
| With ammonium acetate at 160℃; for 1h; | 94% |
-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
42731-38-4
(2Z)-3-hydroxy-1-phenyl-2-propen-1-one sodium salt

-
-
110954-03-5
2-(dicyanomethylene)-1,2-dihydro-6-phenylpyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With piperdinium acetate In ethanol for 0.166667h; Cyclization; Heating; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: carbon disulfide; acetylacetone With tetrabutylammomium bromide; potassium carbonate In 1,4-dioxane at 25℃; for 1h; Stage #2: 1,1,3-tricyano-2-amino-1-propene In 1,4-dioxane at 60℃; for 3h; | 94% |


| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.266667h; Microwave irradiation; | 94% |

-
-
613-84-3
2-hydroxy-5-methylbenzaldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With triethylamine In propan-1-ol at 98℃; for 1h; | 94% |
| With triethylamine In propan-1-ol at 98℃; for 1h; | 94% |

-
-
1076-38-6
4-hydroxy[1]benzopyran-2-one

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

| Conditions | Yield |
|---|---|
| With morpholine In acetonitrile for 4h; Catalytic behavior; Reagent/catalyst; Solvent; Time; Reflux; | 94% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
123-54-6
acetylacetone

-
-
778-50-7
2-(dicyanomethylene)-1,2-dihydro-4,6-dimethylpyridine-3-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 0.166667h; Ambient temperature; | 93% |
| With piperidine In ethanol for 2h; Heating; | 75% |
-
-
18096-70-3
3-(dimethylamino)-1-(4-methoxyphenyl)prop-2-en-1-one

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With piperidine In ethanol Heating; | 93% |
-
-
673-22-3
2-Hydroxy-4-methoxybenzaldehyde

-
-
371-42-6
4-Fluorothiophenol

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 4h; Temperature; Reflux; | 93% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
504-02-9
1,3-cylohexanedione

-
-
86-51-1
2,3-dimethyoxybenzaldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.3h; Microwave irradiation; | 93% |


| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.333333h; Microwave irradiation; | 93% |

-
-
98-03-3
thiophene-2-carbaldehyde

-
-
70254-43-2, 70254-44-3
4-Hydroxy-2-quinolone

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.283333h; Microwave irradiation; | 93% |


| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.266667h; Microwave irradiation; | 93% |

-
-
459-57-4
4-fluorobenzaldehyde

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
504-02-9
1,3-cylohexanedione

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 80℃; for 0.283333h; Microwave irradiation; | 93% |

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
99-61-6
3-nitro-benzaldehyde

-
-
384846-43-9
2-[(5,5-dimethyl-3-oxocyclohexen-1-yl)amino]-4,5,6,7-tetrahydrobenzo[b]thiopene-3-carbonitrile

| Conditions | Yield |
|---|---|
| With piperidine In ethanol for 0.0833333h; Reflux; | 93% |

-
-
501-30-4
5-hydroxy-2-(hydroxymethyl)-4H-pyran-4-one

-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

| Conditions | Yield |
|---|---|
| With potassium fluoride In isopropyl alcohol at 82℃; for 2h; Catalytic behavior; Solvent; Reagent/catalyst; Temperature; Time; | 93% |

2-Amino-1,1,3-tricyanopropene Specification
The 1-Propene-1, 1, 3-tricarbonitrile, 2-amino-, with the CAS registry number of 868-54-2, is also known as 1, 1, 3-Tricyano-2-amino-1-propene and 2-Amino-1, 1, 3-tricyanopropene. Its EINECS registry number is 212-777-5. This chemical's molecular formula is C6H4N4 and molecular weight is 132.12. What's more, its IUPAC name is called 2-Aminoprop-1-ene-1, 1, 3-tricarbonitrile.This chemical is a nootropic drug which mimics the function of nerve growth factor and increases the growth of nerves and tissue regeneration both in isolated tissues and in vivo.
Physical properties about 1-Propene-1, 1, 3-tricarbonitrile, 2-amino- are: (1)ACD/LogP: -0.13; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -0.13; (4)ACD/LogD (pH 7.4): -0.13; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 20.24; (8)ACD/KOC (pH 7.4): 20.24; (9)#H bond acceptors: 4; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 74.61 Å2; (13)Index of Refraction: 1.54; (14)Molar Refractivity: 32.86 cm3; (15)Molar Volume: 104.6 cm3; (16)Polarizability: 13.02×10-24 cm3; (17)Surface Tension: 71.6 dyne/cm; (18)Density: 1.262 g/cm3; (19)Flash Point: 311.8 °C; (20)Enthalpy of Vaporization: 88.28 kJ/mol; (21)Boiling Point: 591.9 °C at 760 mmHg; (22)Melting Point: 171-173 °C (lit. ); (23)Vapour Pressure: 5.54E-14 mmHg at 25°C.
Preparation: this chemical can be prepared by Malononitrile by heating 30 min. The reaction needs reagent KOH and solvent Ethanol. The yield is about 87%.
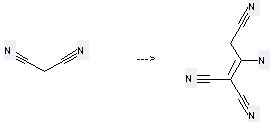
Uses: (1) it is used as releasing oxygen inhibitors of light combination reaction; (2) it can be used to make plastics; (3) it is used to produce other chemicals. For example, it is used to produce C16H7N6(1-)*C4H9N*H(1+) by reaction with Pyrrolidine and Benzylidene-malononitrile.
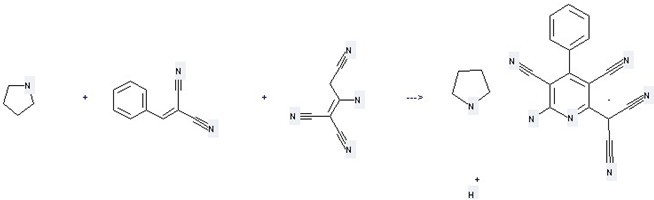
This reaction needs two steps, the reaction conditions are 1.) THF, CHCl3, 35-40 °C, 45 min; 2.) THF, room temp., 1 h, reagent DDQ.
When you are dealing with this chemical, you should be very careful. The dust of this chemical can not be breathed. And you should avoid contacting with skin and eyes for it may cause damage to health.
You can still convert the following datas into molecular structure:
(1) SMILES: N#CCC(=C(/C#N)C#N)\N
(2) InChI: InChI=1/C6H4N4/c7-2-1-6(10)5(3-8)4-9/h1,10H2
(3) InChIKey: BNHGNFYPZNDLAF-UHFFFAOYAY
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intravenous | 180mg/kg (180mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#01433 |
Related Products
- 2-Amino-1-(1,3-benzodioxol-5-yl)ethanone hydrochloride
- 2-Amino-1-(2,4-dichlorophenyl)ethanone hydrochloride
- 2-Amino-1-(2,4-difluorophenyl)ethanone hydrochloride
- 2-Amino-1-(2,5-dimethoxyphenyl)ethanol
- 2-Amino-1-(2-fluorophenyl)ethanol
- 2-amino-1-(3-(trifluoromethyl)phenyl)ethanone hydrochloride
- 2-amino-1-(3,4-dimethylphenyl)ethanol
- 2-Amino-1-(3,4-dimethylphenyl)ethanone hydrochloride
- 2-Amino-1-(3-chlorophenyl)ethanol hydrochloride
- 2-Amino-1-(4'-benzyloxyphenyl)ethanol
- 868543-19-5
- 868551-51-3
- 868551-99-9
- 868552-25-4
- 868561-17-5
- 86857-01-4
- 868-57-5
- 868594-41-6
- 868-59-7
- 868599-73-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View