-
Name
2-Aminonicotinic acid
- EINECS 226-296-3
- CAS No. 5345-47-1
- Article Data25
- CAS DataBase
- Density 1.417 g/cm3
- Solubility Soluble in water
- Melting Point 295-297 °C (dec.)(lit.)
- Formula C6H6N2O2
- Boiling Point 342.311 °C at 760 mmHg
- Molecular Weight 138.126
- Flash Point 160.824 °C
- Transport Information
- Appearance Off-white to light yellow crystalline powder
- Safety 22-24/25-36/37/39-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-Pyridinecarboxylic acid, 2-amino-;Nicotinic acid, 2-amino- (8CI);2-Amino-nicotinic acid;2-animonicotinic acid;2-aminopyridine-3-carboxylic acid;2-Amino-3-pyridinecarboxylic acid;Nicotinic acid, 2-amino-;
- PSA 76.21000
- LogP 0.94320
Synthetic route

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With bromine; sodium hydroxide at 5 - 85℃; for 2.5h; | 98.2% |
-
-
23411-03-2
2-hydroxycarbamoyl-nicotinic acid

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| In formamide at 130 - 150℃; for 20h; | 83.5% |
-
-
88423-10-3
Pyridine-2,3-dicarboxylic acid bis-hydroxyamide

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| In formamide at 130 - 150℃; for 20h; | 80% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; L-2-O-methyl-chiro-inositol; copper(II) acetate monohydrate In 1-methyl-pyrrolidin-2-one at 110℃; for 12h; | 72% |
-
-
2942-59-8
2-chloronicotinic acid

-
A
-
609-71-2
2-hydroxy-3-carboxypyridine

-
B
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With ammonium hydroxide at 155℃; for 20h; | A n/a B 60% |
-
-
24517-64-4
2-amino-3-pyridinecarbonitrile

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 175℃; |

| Conditions | Yield |
|---|---|
| With potassium permanganate Erwaermen des Reaktionsprodukts mit wss. HCl; |
-
-
861045-11-6
2-benzenesulfonylamino-nicotinic acid

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 175℃; |
-
-
1000681-77-5
6H-12H-dipyrido[2,3-b:2',3'-f][1,5]diazocine-5,11-dione

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid
-
-
1000681-77-5
6H-12H-dipyrido[2,3-b:2',3'-f][1,5]diazocine-5,11-dione

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid
-
-
861046-07-3
2-methyl-1,2,3,4-tetrahydro-1,8-naphthyridine

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With sodium hypobromide |

-
A
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With alkaline sodium hypochlorite solution at 80℃; |
-
-
548740-14-3
2-[3,5-bis(trifluoromethyl)phenylsulfonyl]ethyl 2-aminonicotinate

-
A
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
B
-
548740-06-3
3,5-bis(trifluoromethyl)phenyl vinyl sulfone

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In acetone at 20℃; for 12h; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / 30percent H2O2 / acetic acid / 3 h / 90 °C 2: PCl5, POCl3 3: 60 percent / conc. NH4OH / 20 h / 155 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 80 percent / 30percent H2O2 / acetic acid / 3 h / 90 °C 2: 65 percent / POCl3, Et3N / 4 h / 100 °C 3: 60 percent / conc. NH4OH / 20 h / 155 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: PCl5, POCl3 2: 60 percent / conc. NH4OH / 20 h / 155 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 65 percent / POCl3, Et3N / 4 h / 100 °C 2: 60 percent / conc. NH4OH / 20 h / 155 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: NH3; ethanol / 177 °C 2: concentrated aqueous HCl / 175 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: PCl5; POCl3 / 115 - 120 °C 2: NH3; ethanol / 177 °C 3: concentrated aqueous HCl / 175 °C View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: aqueous H2O2; acetic acid 2: PCl5; POCl3 / 115 - 120 °C 3: NH3; ethanol / 177 °C 4: concentrated aqueous HCl / 175 °C View Scheme |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
64-17-5
ethanol

-
-
13362-26-0
2-aminopyridine-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyridin-3-carboxylic acid; ethanol With sulfuric acid for 16h; Heating / reflux; Stage #2: With sodium hydroxide In water pH=8; | 100% |
| With sulfuric acid at 90℃; for 12h; | 98.08% |
| With sulfuric acid at 90℃; for 12h; | 98.08% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; platinum(IV) oxide In ethanol under 1499.7 Torr; for 2h; Ambient temperature; | 100% |
| With hydrogenchloride; PtO2 In methanol | 1.22 g (100%) |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
149-73-5
trimethyl orthoformate

-
-
24410-19-3
3H-pyrido[2,3-d]pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With ammonia In methanol at 105℃; for 8h; | 100% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
2942-58-7
diethyl cyanophosphonate

-
-
2393-23-9
4-methoxy-benzylamine

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide | 100% |

-
-
590-28-3
potassium cyanate

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
21038-66-4
1H-Pyrido[2,3-d]pyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| With water; ammonium chloride at 80 - 200℃; for 2.5h; | 99% |
| In water for 0.00833333h; microwave irradiation; | 95% |
| With acetic acid In polyethyleneglycol (PEG-400) at 20 - 60℃; for 0.166667h; | 78% |
| With ammonium chloride In water at 20 - 200℃; for 2.66667h; |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
52833-94-0
2-amino-5-bromonicotinicacid

| Conditions | Yield |
|---|---|
| With bromine In acetic acid | 99% |
| With bromine In acetic acid | 99% |
| With bromine In acetic acid at 20℃; for 20h; | 98% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
3081-24-1
H-Phe-OEt

-
-
1003023-68-4
(S)-ethyl 2-(2-aminonicotinamido)-3-phenylpropanoate

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide | 98% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
593-51-1
methylamine hydrochloride

-
-
870997-87-8
2-amino-N-methyl-nicotinamide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 98% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
99-61-6
3-nitro-benzaldehyde

-
-
1608494-18-3
3-(tert-butylamino)-2-(3-nitrophenyl)imidazo[1,2-a]pyridine-8-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 3h; | 98% |

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
22227-25-4
3-(trifluoromethyl)benzene-1-carbohydrazide

| Conditions | Yield |
|---|---|
| With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; triethylamine In N,N-dimethyl-formamide at 20℃; | 98% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
57-13-6
urea

-
-
21038-66-4
2,4-dihydroxy-pyrido[2,3-d]pyrimidine

| Conditions | Yield |
|---|---|
| at 280℃; | 97% |
| at 195℃; for 1.5h; | 76% |
| at 210℃; for 0.25h; | 63.3% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
2450-71-7
Propargylamine

-
-
1064689-94-6
3-(prop-2-ynyl)pyrido[2,3-d]pyrimidin-4(3H)-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyridin-3-carboxylic acid; N,N-dimethyl-formamide dimethyl acetal In N,N-dimethyl-formamide at 100℃; under 760.051 Torr; for 0.25h; Microwave irradiation; Inert atmosphere; Green chemistry; Stage #2: Propargylamine With acetic acid at 100℃; under 760.051 Torr; for 0.25h; Inert atmosphere; Microwave irradiation; Green chemistry; | 97% |

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
931-53-3
Cyclohexyl isocyanide

-
-
104-87-0
4-methyl-benzaldehyde

-
-
1608494-14-9
3-(cyclohexylamino)-2-(4-methylphenyl)imidazo[1,2-a]pyridine-8-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 3h; | 97% |


| Conditions | Yield |
|---|---|
| With sodium azide In neat (no solvent) at 90℃; for 0.833333h; Reagent/catalyst; Green chemistry; | 97% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
108-24-7
acetic anhydride

-
-
3809-93-6
2-Methyl-4H-pyrido<2,3-d><3,1>oxazin-4-one

| Conditions | Yield |
|---|---|
| at 170℃; for 1h; | 96% |
| at 165 - 170℃; for 1h; | 90% |
| for 2h; Inert atmosphere; Reflux; | 79% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
23612-57-9
(2-amino-3-pyridinyl)methanol

| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyridin-3-carboxylic acid With lithium aluminium tetrahydride In tetrahydrofuran for 18h; Heating / reflux; Stage #2: With sodium hydroxide In tetrahydrofuran; water for 0.25h; | 96% |
| With lithium aluminium tetrahydride In tetrahydrofuran for 18h; Heating; | 96% |
| With lithium aluminium tetrahydride In tetrahydrofuran at 0℃; for 18h; Reflux; | 96% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
57-13-6
urea

-
-
21038-66-4
1H-Pyrido[2,3-d]pyrimidine-2,4-dione

| Conditions | Yield |
|---|---|
| In water for 0.075h; microwave irradiation; | 95% |
| Stage #1: 2-aminopyridin-3-carboxylic acid; urea at 180 - 210℃; for 1.5h; Stage #2: With sodium hydroxide In water at 50℃; | 82% |
| Stage #1: 2-aminopyridin-3-carboxylic acid; urea at 180 - 210℃; for 0.25h; Stage #2: With sodium hydroxide In water at 20 - 55℃; | 76% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
13438-65-8
2-aminopyridine-3-carboxamide

| Conditions | Yield |
|---|---|
| With ammonium chloride; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; for 12h; | 95% |
| With ammonium chloride; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In N,N-dimethyl-formamide at 20℃; | 89% |
| Stage #1: 2-aminopyridin-3-carboxylic acid With 1,1'-carbonyldiimidazole In tetrahydrofuran for 12h; Inert atmosphere; Stage #2: With ammonia In tetrahydrofuran Inert atmosphere; | 77.7% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
14667-47-1
methyl 2-aminonicotinate

| Conditions | Yield |
|---|---|
| In methanol; toluene at 0℃; for 0.5h; | 95% |
| In methanol; diethyl ether; toluene at 0℃; for 0.75h; Inert atmosphere; | 90% |
| In methanol; hexane for 0.5h; |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
13362-26-0
2-aminopyridine-3-carboxylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 16h; Reflux; | 95% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
119072-55-8, 7188-38-7
tert-butylisonitrile

-
-
552-89-6
2-nitro-benzaldehyde

-
-
1608494-20-7
3-(tert-butylamino)-2-(2-nitrophenyl)imidazo[1,2-a]pyridine-8-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 3h; | 95% |

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
931-53-3
Cyclohexyl isocyanide

-
-
613-45-6
2,4-Dimethoxybenzaldehyde

| Conditions | Yield |
|---|---|
| In ethanol at 60℃; for 6h; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: 2-aminopyridin-3-carboxylic acid With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 2h; Stage #2: α-bromoacetophenone In N,N-dimethyl-formamide at 50℃; for 3h; | 94% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
14667-47-1
methyl 2-aminonicotinate

| Conditions | Yield |
|---|---|
| With triethylamine In methanol at 20℃; for 1h; | 94% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
57260-71-6
1-t-Butoxycarbonylpiperazine

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine In N,N-dimethyl-formamide at 60℃; for 2h; Inert atmosphere; | 93.4% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 0 - 60℃; under 760.051 Torr; for 1.5h; Inert atmosphere; Microwave irradiation; | 93% |
| With thionyl chloride at 0 - 80℃; for 3h; | 86% |
| With sulfuric acid at 80℃; for 7h; | 63% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
52963-33-4
2-amino-5-bromonicotinic acid hydrobromide

| Conditions | Yield |
|---|---|
| With bromine In acetic acid at 0 - 20℃; for 48h; | 93% |
| With bromine; acetic acid at 20℃; for 1h; | 93% |
| With bromine; acetic acid at 20℃; Cooling with ice; | 91% |
-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
931-53-3
Cyclohexyl isocyanide

-
-
99-61-6
3-nitro-benzaldehyde

-
-
1608494-16-1
3-(cyclohexylamino)-2-(3-nitrophenyl)imidazo[1,2-a]pyridine-8-carboxylic acid

| Conditions | Yield |
|---|---|
| In ethanol at 50℃; for 3h; | 93% |

-
-
5345-47-1
2-aminopyridin-3-carboxylic acid

-
-
439277-55-1
2-chloro-3-(4-chlorophenyl)-1,8-naphthyridine

-
-
1075254-10-2
C20H11ClN4O

| Conditions | Yield |
|---|---|
| With acetic acid for 0.075h; microwave irradiation; | 92% |
2-Aminonicotinic acid Chemical Properties
IUPAC Name: 2-Aminopyridine-3-carboxylic acid
Synonyms of 2-Amino nicotinic acid (CAS NO.5345-47-1): 2-Amino-3-pyridinecarboxylic acid ; 3-Pyridinecarboxylic acid, 2-amino- ; Nicotinic acid, 2-amino- (8CI)
CAS NO: 5345-47-1
Molecular Formula: C6H6N2O2
Molecular Weight:138.12
Molecular Structure: 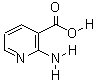
EINECS: 226-296-3
H bond acceptors: 4
H bond donors: 3
Freely Rotating Bonds: 1
Polar Surface Area: 42.43 Å2
Index of Refraction: 1.649
Molar Refractivity: 35.51 cm3
Molar Volume: 97.4 cm3
Surface Tension: 77.7 dyne/cm
Density: 1.417 g/cm3
Flash Point: 160.8 °C
Enthalpy of Vaporization: 61.85 kJ/mol
Boiling Point: 342.3 °C at 760 mmHg
Vapour Pressure: 2.92E-05 mmHg at 25°C
Melting point: 295-297 °C (dec.)(lit.)
Water Solubility: soluble
Appearance: Off-white to light yellow crystalline powder
Product Categories of 2-Amino nicotinic acid (CAS NO.5345-47-1): AMINOACID;Amines;blocks;Carboxes;Pyridines;Amino Acids and Derivatives;Heterocycles;Aromatic Carboxylic Acids, Amides, Anilides, Anhydrides & Salts;Carboxylic Acids;Pyridine;Pyridines derivates;Carboxylic Acids
2-Aminonicotinic acid Safety Profile
Safety Information about 2-Amino nicotinic acid (CAS NO.5345-47-1):
Hazard Codes:  Xi
Xi
The Risk Statements: 36/37/38
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements: 22-24/25-36/37/39-26
22: Do not breathe dust
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
24/25: Avoid contact with skin and eyes
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection.
WGK Germany:3
Hazard Note:Irritant
TSCA:T
2-Aminonicotinic acid Specification
General Information: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion.
Extinguishing Media: Use water spray, dry chemical, carbon dioxide, or chemical foam.
Handling: Avoid breathing dust, vapor, mist, or gas. Avoid contact with skin and eyes. Avoid ingestion and inhalation.
Storage: Store in a cool, dry place. Store in a tightly closed container.
Related Products
- 2-Aminonicotinic acid
- 5345-53-9
- 5345-54-0
- 5345-70-0
- 534571-98-7
- 534573-39-2
- 53458-16-5
- 53458-31-4
- 5345-89-1
- 534595-51-2
- 534595-53-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View