-
Name
2-Benzylacrylic acid
- EINECS 219-673-9
- CAS No. 5669-19-2
- Article Data58
- CAS DataBase
- Density 1.12 g/cm3
- Solubility
- Melting Point 66-68 °C
- Formula C10H10O2
- Boiling Point 326.1 °C at 760 mmHg
- Molecular Weight 162.188
- Flash Point 230.1 °C
- Transport Information
- Appearance White Solid
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Hydrocinnamicacid, a-methylene- (6CI,8CI);2-Methylene-3-phenylpropanoicacid;NSC 192640;a-Benzylacrylic acid;a-Methylenebenzenepropanoicacid;
- PSA 37.30000
- LogP 1.86990
Synthetic route
-
-
20593-63-9
2-Benzylacrylic acid ethyl ester

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With lithium hydroxide In tetrahydrofuran; water for 15h; Heating; | 100% |
| With ethanol; sodium hydroxide In dichloromethane at 10 - 20℃; for 3.5h; Time; Reflux; | 92.8% |
| With potassium hydroxide In methanol for 18h; | 80% |

| Conditions | Yield |
|---|---|
| With diethylamine for 5h; Mannich reaction; | 98% |
| With diethylamine In ethyl acetate at 0℃; for 3h; Reflux; | 90% |
| With diethylamine In ethyl acetate for 4h; Inert atmosphere; Reflux; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-benzylmalonic acid With formaldehyd; diethylamine In ethyl acetate at 0 - 20℃; for 1.5h; Heating / reflux; Stage #2: With hydrogenchloride; water at 10℃; | 90% |
| Stage #1: 2-benzylmalonic acid With formaldehyd; diethylamine In ethyl acetate Stage #2: With potassium hydroxide | |
| Multi-step reaction with 2 steps 1: concentrated ammonia View Scheme |
-
-
109481-98-3
(Z)-3-Phenyl-2-trimethylsilanylmethyl-acrylic acid ethyl ester

-
A
-
5669-19-2
2-Benzyl-2-propenoic acid

-
B
-
1895-97-2
2-methyl-3-phenylacrylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water In methanol for 3h; Reflux; | A 12% B 87% |

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 100℃; for 2h; | 80% |
-
-
358382-80-6
5-benzyl-5-hydroxymethyl-2,2-dimethyl-[1,3]dioxane-4,6-dione

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide In water; acetic acid for 2h; Heating; | 76% |

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With hydrogen bromide; acetic acid at 90℃; for 18h; | 72.4% |
| With hydrogen bromide; acetic acid In water at 80℃; for 72h; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol | 66% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 20h; Ambient temperature; | 65% |
| With potassium hydroxide In ethanol Heating; | |
| With lithium hydroxide monohydrate; water In tetrahydrofuran at 70℃; for 3h; |
-
-
109481-98-3
(Z)-3-Phenyl-2-trimethylsilanylmethyl-acrylic acid ethyl ester

-
A
-
1039704-09-0
(2Z)-3-phenyl-2-[(trimethylsilyl)methyl]prop 2-enoic acid

-
B
-
5669-19-2
2-Benzyl-2-propenoic acid

-
C
-
1895-97-2
2-methyl-3-phenylacrylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide; water In methanol for 0.333333h; Reflux; | A 19% B 13% C 64% |

-
-
28769-48-4
α-benzylacrylonitrile

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In water at 130℃; for 15h; | 62% |
| With hydrogenchloride | |
| With potassium hydroxide In 2-ethoxy-ethanol Heating; |
-
-
30457-88-6
2-benzyl-2-propenal

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With sodium chlorite; sodium phosphate In water; tert-butyl alcohol at 20℃; for 0.5h; | 36% |
-
-
97-65-4
2-methylenesuccinic acid

-
-
100-34-5
benzene diazonium chloride

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With water; sodium acetate; copper dichloride Bestrahlen des Reaktionsprodukts in Gegenwart von Brom in Aether und CHCl3; |
-
-
861356-96-9
benzyl-piperidinomethyl-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid
-
-
408308-23-6
aminomethyl-benzyl-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With water |
-
-
69858-92-0
benzyl-methoxymethyl-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride | |
| With sulfuric acid at 170 - 175℃; |
-
-
408308-33-8
benzyl-methylaminomethyl-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid
-
-
24643-58-1
2-benzyl-2-carboxy-3-(dimethylamino)propionic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| Thermolysis; | |
| beim Schmelzen im Vakuum; |

-
-
5669-19-2
2-Benzyl-2-propenoic acid
-
-
855653-03-1
benzyl-methanesulfonylmethyl-malonic acid diethyl ester

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
871882-45-0
2,2'-dibenzyl-2,2'-(2-aza-propanediyl)-di-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid
-
-
78763-98-1
2-(1-Benzyl-vinyl)-4,4-dimethyl-4,5-dihydro-oxazole

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; acetic acid Heating; Yield given; |

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With diethyl ether; magnesium |

-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water; acetic acid |
-
-
7647-01-0
hydrogenchloride

-
-
69858-92-0
benzyl-methoxymethyl-malonic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid
-
-
7647-01-0
hydrogenchloride

-
-
69858-85-1
benzyl-methoxymethyl-malonic acid diethyl ester

-
-
7732-18-5
water

-
-
64-19-7
acetic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid


| Conditions | Yield |
|---|---|
| With 50 mmol/l Tris*HCl buffer; Bacillus subtilis chorismate mutase at 30℃; pH=8.6; Product distribution; Further Variations:; Reagents; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: KOH / aq. methanol 2.1: p-formaldehyde; diethylamine / ethyl acetate 2.2: aq. KOH View Scheme | |
| Multi-step reaction with 2 steps 1: KOH / ethanol / 4 h / 0 - 20 °C 2: Et3N / H2O / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: water; potassium hydroxide / ethanol / 10 h / 20 °C 2: dimethyl amine / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide; water / 5 h / Reflux 2: diethylamine / ethyl acetate / 3 h / 0 °C / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: sodium hydroxide / methanol; water / 20 °C / Inert atmosphere 2: diethylamine / ethyl acetate / 4 h / Inert atmosphere; Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 0 - 20℃; for 18h; | 100% |
| With boron trifluoride diethyl etherate for 14h; Heating; | 94% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
3070-71-1
methyl 2-benzylacrylate

| Conditions | Yield |
|---|---|
| In methanol; hexane; benzene at 20℃; for 0.5h; | 100% |
| 89% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
88320-94-9
2-benzylpropenoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 1.5h; | 100% |
| With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane at 0 - 15℃; for 2h; Solvent; Temperature; Reagent/catalyst; Industrial scale; | 99.4% |
| With thionyl chloride at 20℃; for 3h; | 96% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
507-09-5
thioacetic acid

-
-
91702-98-6
3-acetylthio-2-benzylpropanoic acid

| Conditions | Yield |
|---|---|
| In dichloromethane for 1152h; | 99% |
| In tetrahydrofuran Heating; | 96% |
| In benzene Heating; | 77% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
14367-67-0
2-methyl-3-phenylpropionic acid

| Conditions | Yield |
|---|---|
| With C54H72IrNP(1+)*C32H12BF24(1-); hydrogen; caesium carbonate In methanol at 45℃; under 4500.45 Torr; for 0.25h; Pressure; Glovebox; | 99% |
| With disodium hydrogen phosphite pentahydrate; phosphite dehydrogenase; water; nicotinamide adenine dinucleotide phosphate; ene-reductase 36 at 25℃; pH=6.5; Catalytic behavior; Reagent/catalyst; pH-value; Temperature; Enzymatic reaction; enantioselective reaction; | 99% |
| With C32H12BF24(1-)*C54H71IrNP(1+); hydrogen; caesium carbonate In methanol at 45℃; for 4h; optical yield given as %ee; enantioselective reaction; | 98% |
| Stage #1: 2-Benzyl-2-propenoic acid With hydrogen; caesium carbonate; [(Sa-DTB-SIPHOX)Ir(COD)]BARF In methanol at 20℃; under 4500.45 Torr; for 12h; Stage #2: With hydrogenchloride In water pH=< 3; Product distribution / selectivity; | 94% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
1009-67-2, 5628-72-8, 14367-54-5, 14367-67-0
2-methyl-3-phenylpropionic acid

| Conditions | Yield |
|---|---|
| With C48H50Cl4N2O2P2Ru3; hydrogen; sodium hydrogencarbonate In methanol at 20℃; under 3750.38 Torr; for 24h; Autoclave; enantioselective reaction; | 99% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
86396-02-3
2-(phenyl-λ3-iodaneylidene)cyclohexane-1,3-dione

-
-
1000697-64-2
3-benzyl-7,8-dihydro-2H-chromene-2,5(6H)-dione

| Conditions | Yield |
|---|---|
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; sodium acetate at 80℃; for 12h; Catalytic behavior; Mechanism; Temperature; Reagent/catalyst; Sealed tube; | 99% |
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; sodium acetate at 80℃; for 9h; Temperature; Reagent/catalyst; | 99% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
86396-05-6
(2,6-dioxo-4-phenylcyclohexyl)phenyliodonium inner salt

| Conditions | Yield |
|---|---|
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; sodium acetate at 80℃; for 12h; | 98% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
507-09-5
thioacetic acid

-
-
149603-85-0
2-acetylthio-3-phenyl-propionic acid

| Conditions | Yield |
|---|---|
| In CaCl2 | 95% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
82860-53-5
2-hydroxy-N,N-diiso-propylbenzamide

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In toluene at 80℃; for 9h; | 95% |

| Conditions | Yield |
|---|---|
| With bis[dichloro(pentamethylcyclopentadienyl)iridium(III)]; tetrabutylammonium acetate In methanol at 60℃; for 12h; Electrochemical reaction; | 95% |
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; silver carbonate at 80℃; for 8h; | 60% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; [Ir(ppy)2(dtbbpy)]PF6 In acetone at 0 - 40℃; for 6h; Michael Addition; Inert atmosphere; Irradiation; | 93% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With Cp*Rh(OAc)2·H2O In 1,2-dichloro-ethane at 60℃; for 9h; stereoselective reaction; | 92% |

| Conditions | Yield |
|---|---|
| With (pentamethylcyclopentadienyl)*Rh(OAc)2 In 1,2-dichloro-ethane at 60℃; for 9h; | 92% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With Cp*Rh(OAc)2·H2O In 1,2-dichloro-ethane at 60℃; for 9h; stereoselective reaction; | 91% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With Cp*Rh(OAc)2·H2O In 1,2-dichloro-ethane at 60℃; for 9h; stereoselective reaction; | 91% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With (pentamethylcyclopentadienyl)*Rh(OAc)2 In 1,2-dichloro-ethane at 60℃; for 9h; | 91% |

| Conditions | Yield |
|---|---|
| With phenylsilane; C12H23N2O2P In tetrahydrofuran at 23℃; for 2h; | 91% |
| With hydrogen |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
865703-11-3
2-benzyl-3-(hydroxyhydrophosphoryl)propanoic acid

| Conditions | Yield |
|---|---|
| With bis(trimethylsilyl)phosphonite In dichloromethane at 0 - 20℃; for 25h; Inert atmosphere; | 91% |
-
-
507-09-5
thioacetic acid

-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
91702-98-6
3-acetylthio-2-benzylpropanoic acid

| Conditions | Yield |
|---|---|
| at 100℃; for 2h; | 90% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With Cp*Rh(OAc)2·H2O In 1,2-dichloro-ethane at 60℃; for 9h; stereoselective reaction; | 90% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With (pentamethylcyclopentadienyl)*Rh(OAc)2 In 1,2-dichloro-ethane at 60℃; for 9h; | 90% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
86396-03-4
(2,6-dioxo-4-methylcyclohexyl)phenyliodonium inner salt

| Conditions | Yield |
|---|---|
| With dichloro(pentamethylcyclopentadienyl)rhodium (III) dimer; sodium acetate at 80℃; for 12h; | 90% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

-
-
7480-35-5, 13286-59-4, 74165-73-4, 126456-43-7, 136030-00-7, 140632-19-5, 140632-20-8
(1S,2R)-1-amino-2-indanol

-
-
872703-52-1
2-benzyl-N-[(1S,2R)-2-hydroxy-indan-1-yl]acrylamide

| Conditions | Yield |
|---|---|
| With (benzotriazo-1-yloxy)tris(dimethylamino)phosphonium hexafluorophosphate; triethylamine | 89% |
| Stage #1: 2-Benzyl-2-propenoic acid With 4-methyl-morpholine; benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In ethyl acetate at 20℃; for 0.5h; Stage #2: (1S,2R)-1-amino-2-indanol In ethyl acetate at 20℃; | 68% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With Cp*Rh(OAc)2·H2O In 1,2-dichloro-ethane at 60℃; for 9h; stereoselective reaction; | 88% |
-
-
5669-19-2
2-Benzyl-2-propenoic acid

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; [Ir(ppy)2(dtbbpy)]PF6 In acetone at 0 - 40℃; for 5h; Michael Addition; Inert atmosphere; Irradiation; | 87% |
2-Benzylacrylic acid Specification
The CAS registry number of 2-Benzyl acrylic acid is 5669-19-2. The IUPAC name is 2-benzylprop-2-enoic acid. In addition, the molecular formula is C10H10O2. What's more, it is a kind of white solid and belongs to the classes of Racecadotril; Aliphatics; Aromatics. It can be used as pharmaceutical intermediates and raw materials in organic synthesis. Besides, it should be stored in sealed container, and put in a cool and dry place.
Physical properties about this chemical are: (1)ACD/LogP: 2.50; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 1.44; (4)ACD/BCF (pH 5.5): 4.06; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 47.41; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 26.3 Å2; (12)Index of Refraction: 1.551; (13)Molar Refractivity: 46.2 cm3; (14)Molar Volume: 144.7 cm3; (15)Polarizability: 18.31 ×10-24cm3; (16)Surface Tension: 42.8 dyne/cm; (17)Density: 1.12 g/cm3; (18)Flash Point: 230.1 °C; (19)Enthalpy of Vaporization: 59.99 kJ/mol; (20)Boiling Point: 326.1 °C at 760 mmHg; (21)Vapour Pressure: 8.97E-05 mmHg at 25°C.
Preparation of 2-Benzyl acrylic acid: it can be prepared by a-Benzylacrylsaeuremethylester. This reaction will need reagent aq. NaOH and solvent methanol. The reaction time is 20 hours with ambient temperature. The yield is about 65%.
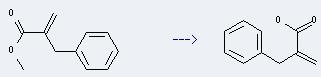
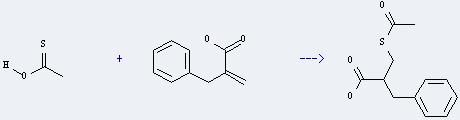
Uses of 2-Benzyl acrylic acid: it can react with thioacetic acid to get 2-acetylsulfanylmethyl-3-phenyl-propionic acid. This reaction is a kind of addition reaction. It will need solvent tetrahydrofuran. The reaction time is 18 hours at reaction temperature of 50 °C.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O)\C(=C)Cc1ccccc1
(2)InChI: InChI=1/C10H10O2/c1-8(10(11)12)7-9-5-3-2-4-6-9/h2-6H,1,7H2,(H,11,12)
(3)InChIKey: RYNDYESLUKWOEE-UHFFFAOYAD
Related Products
- 2-Benzylacrylic acid
- 56691-99-7
- 56692-00-3
- 56692-02-5
- 566939-85-3
- 56-69-9
- 56700-70-0
- 56701-24-7
- 56704-25-7
- 56705-79-4
- 56705-83-0
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View