-
Name
BROMOHYDROQUINONE
- EINECS 209-516-2
- CAS No. 583-69-7
- Article Data53
- CAS DataBase
- Density 1.844 g/cm3
- Solubility
- Melting Point 112-116 °C(lit.)
- Formula C6H5BrO2
- Boiling Point 278.3 °C at 760 mmHg
- Molecular Weight 189.008
- Flash Point 122.1 °C
- Transport Information
- Appearance beige to brown fine crystalline powder
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Hydroquinone,bromo- (6CI,7CI,8CI);1-Bromo-2,5-dihydroxybenzene;2-Bromo-1,4-benzenediol;2-Bromo-1,4-dihydroxybenzene;2-Bromo-1,4-hydroquinone;2-Bromohydroquinone;2-Bromoquinol;Bromohydroquinone;NSC 3977;
- PSA 40.46000
- LogP 1.86030
Synthetic route

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; potassium bromide In water; acetonitrile | 97% |
| With o-xylylene bis(triethylammonium tribromide) In acetonitrile at 20℃; for 0.0833333h; regioselective reaction; | 97% |
| With bromine In chloroform at 0 - 25℃; for 3.25h; | 92% |

| Conditions | Yield |
|---|---|
| With sodium azide In water; acetone at 20℃; | 95% |
| With 1,4-dihydronicotinamide adenine dinucleotide In water; acetonitrile at 30℃; Rate constant; pH=7.0; |
-
-
52376-16-6
2-bromo-1,4-phenylene diacetate

-
-
583-69-7
2-bromobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| With sodium hydroxide for 12h; Ambient temperature; | 90% |
| With sodium hydroxide at 20℃; |

| Conditions | Yield |
|---|---|
| With oxygen; copper diacetate; trifluoroacetic acid; lithium bromide In acetonitrile at 80℃; under 760.051 Torr; for 10h; Sealed tube; | 88% |
-
-
52376-17-7
1,4-O-di-propanoylbromohydroquinone

-
-
583-69-7
2-bromobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B In di-isopropyl ether; isopropyl alcohol at 45℃; for 1h; Enzymatic reaction; regioselective reaction; | 86% |

| Conditions | Yield |
|---|---|
| With Diethyl 2-bromomalonate at 100℃; for 48h; Product distribution; Further Variations:; Reagents; | A 80% B 11% |
-
-
108-86-1
bromobenzene

-
A
-
5926-51-2
bromomaleic anhydride

-
B
-
591-20-8
3-Bromophenol

-
C
-
95-56-7
2-hydroxybromobenzene

-
D
-
583-69-7
2-bromobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| With Fe2(N,N-bis(pyridin-2-ylmethyl)prop-2-yn-1-amine)2(μ2-Cl)2Cl2; dihydrogen peroxide In acetonitrile at 70℃; for 2h; | A 6% B 49% C 29% D 23% |


| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate | |
| With edetate disodium; L-proline; diothiothreitol In dimethyl sulfoxide; glycerol at 28℃; for 24h; pH=7.2; Microbiological reaction; sodium phosphate buffer; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide |

| Conditions | Yield |
|---|---|
| With tetrachloromethane; bromine |

| Conditions | Yield |
|---|---|
| With diethyl ether; hydrogen bromide | |
| With chloroform; hydrogen bromide | |
| With hydrogen bromide |

| Conditions | Yield |
|---|---|
| With hydrogen bromide |
-
-
150-78-7
1,4-dimethoxybezene

-
A
-
17332-11-5
2-bromo-4-methoxyphenol

-
B
-
583-69-7
2-bromobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| With hydrogen bromide; fluorosulphonic acid; lead dioxide 1.)-72 deg C, 3 h; Yield given. Multistep reaction. Yields of byproduct given; |
-
-
60-29-7
diethyl ether

-
-
67-66-3
chloroform

-
-
7726-95-6
bromine

-
-
123-31-9
hydroquinone

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
67-66-3
chloroform

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
-
106-51-4
p-benzoquinone

-
A
-
583-69-7
2-bromobenzene-1,4-diol

-
B
-
14753-51-6
2,5-dibromohydroquinone

-
-
7732-18-5
water

-
-
10035-10-6, 12258-64-9
hydrogen bromide

-
-
106-51-4
p-benzoquinone

-
A
-
583-69-7
2-bromobenzene-1,4-diol

-
B
-
14753-51-6
2,5-dibromohydroquinone

| Conditions | Yield |
|---|---|
| bei laengerer Einwirkung; |

-
-
2973-78-6
3-bromo-4-hydroxybenzylaldehyde

-
-
7722-84-1
dihydrogen peroxide

-
-
583-69-7
2-bromobenzene-1,4-diol


| Conditions | Yield |
|---|---|
| at 160℃; im Rohr; |

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide In acetonitrile at 30℃; for 0.2h; UV-irradiation; Title compound not separated from byproducts.; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 96 percent / ZnBr2 / 3 h / 100 °C 2: aq. NaOH / 20 °C View Scheme |

| Conditions | Yield |
|---|---|
| With edetate disodium; L-proline; diothiothreitol In dimethyl sulfoxide; glycerol at 28℃; for 24h; pH=7.2; Microbiological reaction; sodium phosphate buffer; |
-
-
108-86-1
bromobenzene

-
-
127-17-3
2-oxo-propionic acid

-
A
-
583-69-7
2-bromobenzene-1,4-diol

-
B
-
38739-13-8
(2S)-2-amino-3-(3-bromo-4-hydroxyphenyl)propanoic acid

| Conditions | Yield |
|---|---|
| With glucose dehydrogenase; D-glucose; pyridoxal 5'-phosphate; P450 monooxygenase BM3 variant M2; tyrosine phenol lyase mutant M379V; ammonia; oxygen; NADPH; catalase In aq. phosphate buffer; dimethyl sulfoxide at 20℃; for 6h; pH=8; Enzymatic reaction; enantioselective reaction; | A n/a B n/a |

-
-
108-86-1
bromobenzene

-
A
-
64-18-6
formic acid

-
B
-
583-69-7
2-bromobenzene-1,4-diol

-
C
-
120-80-9
benzene-1,2-diol

-
D
-
123-31-9
hydroquinone

| Conditions | Yield |
|---|---|
| With iron-tungstate oxide capsule; air In water-d2 at 20℃; under 750.075 Torr; for 24h; Electrolysis; |

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
107-30-2
chloromethyl methyl ether

-
-
131136-47-5
2-Bromo-1,4-bis(methoxymethoxy)benzene

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; Inert atmosphere; | 100% |
| Stage #1: 2-bromobenzene-1,4-diol With sodium hydride In N,N-dimethyl-formamide at 0 - 20℃; for 0.5h; Stage #2: chloromethyl methyl ether In DMF (N,N-dimethyl-formamide) at 0 - 20℃; for 0.5h; | 83% |
| Stage #1: 2-bromobenzene-1,4-diol With ethylmagnesium bromide In tetrahydrofuran; diethyl ether at 20℃; for 1h; Stage #2: chloromethyl methyl ether In tetrahydrofuran; diethyl ether at 20 - 55℃; Further stages.; | 81% |
| With ethylmagnesium bromide 1.) THF, room temperature, 1 h, 2.) 20-25 deg C, overnight, then 50-55 deg C, 1 h; Yield given. Multistep reaction; | |
| With ethylmagnesium bromide 1.) THF, r.t., 6 h; 2.) THF; r.t., 12 h; 50 - 55 degC, 1 h; Yield given. Multistep reaction; |
-
-
75-77-4
chloro-trimethyl-silane

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
67289-10-5
1,4-bis(trimethylsilyloxy)-2-bromobenzene

| Conditions | Yield |
|---|---|
| With triethylamine for 1h; | 100% |

| Conditions | Yield |
|---|---|
| With oxygen; Nitrogen dioxide In dichloromethane at -10℃; for 10h; | 99% |
| With oxygen; Nitrogen dioxide In dichloromethane at -10℃; for 10h; | 99% |
| With tetrabutylammonium chromate In dichloromethane for 0.5h; Reflux; | 99% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
999-97-3
1,1,1,3,3,3-hexamethyl-disilazane

-
-
67289-10-5
1,4-bis(trimethylsilyloxy)-2-bromobenzene

| Conditions | Yield |
|---|---|
| for 15h; Heating; | 99% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
79271-56-0
Triethylsilyl trifluoromethanesulfonate

-
-
387400-90-0
2-bromo-1,4-bis-triethylsilanyloxy-benzene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide at 20℃; for 0.5h; | 98% |
| With 1H-imidazole In N,N-dimethyl-formamide at 0 - 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; tetrabutyl-ammonium chloride; sodium carbonate In N,N-dimethyl-formamide at 100℃; for 5h; Heck reaction; | 98% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; tetrabutyl-ammonium chloride; sodium carbonate In N,N-dimethyl-formamide at 100℃; for 5h; Heck reaction; | 96% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
108-24-7
acetic anhydride

-
-
52376-16-6
2-bromo-1,4-phenylene diacetate

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; | 95% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
78018-59-4
1,4-Bis(tert-butyldimethysiloxy)-2-bromobenzene

| Conditions | Yield |
|---|---|
| With 1H-imidazole In N,N-dimethyl-formamide for 15h; Ambient temperature; | 95% |
-
-
5292-43-3
bromoacetic acid tert-butyl ester

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
1191430-29-1
C18H25BrO6

| Conditions | Yield |
|---|---|
| Stage #1: 2-bromobenzene-1,4-diol With caesium carbonate In acetone at 20℃; for 0.5h; Inert atmosphere; Stage #2: bromoacetic acid tert-butyl ester In acetone at 70℃; for 1h; Inert atmosphere; | 94% |
-
-
16939-57-4
(E)-1-Phenyl-1,3-butadiene

-
-
583-69-7
2-bromobenzene-1,4-diol

| Conditions | Yield |
|---|---|
| With palladium diacetate; tetrabutyl-ammonium chloride; sodium carbonate In N,N-dimethyl-formamide at 100℃; for 5h; Heck reaction; | 92% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
112-89-0
1-Bromooctadecane

-
-
642476-84-4
1-bromo-2,5-dioctadecyloxybenzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20 - 30℃; for 8h; | 92% |
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 2h; | 90% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
126689-00-7
2-methyl-1-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)-1-propene

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran; water at 80℃; for 48h; Suzuki Coupling; Inert atmosphere; | 92% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
100-39-0
benzyl bromide

-
-
2237-21-0
(2-bromo-1,4-phenylene)bis(oxy)bis(methylene)dibenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone Reflux; | 91% |
| With potassium carbonate In acetone for 15h; Reflux; | 89% |
| With potassium carbonate In acetone for 15h; Alkylation; Heating; | 88% |
-
-
111-25-1
1-bromo-hexane

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
202798-00-3
2-bromo-1,4-bis(hexyloxy)benzene

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In N,N-dimethyl-formamide at 110℃; for 24h; Inert atmosphere; | 90% |
| With 18-crown-6 ether; potassium carbonate In N,N-dimethyl-formamide at 110℃; for 24h; Inert atmosphere; | 90% |
| With potassium carbonate In acetone for 72h; Reflux; | 40% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
143-15-7
1-dodecylbromide

-
-
171368-73-3
1-bromo-2,5-didodecyloxybenzene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In N,N-dimethyl-formamide at 100℃; for 16h; Alkylation; | 88% |
| With sodium hydroxide In N,N-dimethyl-formamide for 48h; Williamson reaction; Heating; | 62% |

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 20℃; for 15h; | 88% |
-
-
111-83-1
1-bromo-octane

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
194204-71-2
2-bromo-1,4-bis(octyloxy)benzene

| Conditions | Yield |
|---|---|
| With potassium hydroxide In dimethyl sulfoxide at 20℃; for 22h; | 87% |
| Stage #1: 2-bromobenzene-1,4-diol With potassium carbonate In acetonitrile at 20℃; for 1h; Stage #2: 1-bromo-octane In acetonitrile for 18h; Reflux; | 77% |
| With potassium carbonate In butanone for 24h; Reflux; Inert atmosphere; | 3.71 g |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol Heating; | 86% |

| Conditions | Yield |
|---|---|
| With sodium hydride | 83% |

-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
1372769-43-1
4'-(trifluorovinyloxy)biphenyl-2,5-diol

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran; water at 80℃; for 12h; Suzuki coupling; Inert atmosphere; | 80% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
157945-83-0
(E)-2-(3,3-dimethylbut-1-en-1-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

| Conditions | Yield |
|---|---|
| With tetrakis(triphenylphosphine) palladium(0); potassium carbonate In tetrahydrofuran; water at 80℃; for 15h; Inert atmosphere; | 79% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
69739-34-0
t-butyldimethylsiyl triflate

-
-
78018-59-4
1,4-Bis(tert-butyldimethysiloxy)-2-bromobenzene

| Conditions | Yield |
|---|---|
| With triethylamine In chloroform | 72% |
-
-
583-69-7
2-bromobenzene-1,4-diol

-
-
62921-74-8
2-(2-(2-methoxyethoxy)ethoxy)ethyl p-toluenesulfonate

-
-
913544-48-6
1,4-bis((triethylene glycol monomethyl ether)oxy)-2-bromobenzene

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In butanone for 48h; Heating; | 68.5% |
2-Bromohydroquinone Consensus Reports
Reported in EPA TSCA Inventory.
2-Bromohydroquinone Specification
This chemical is called 2-Bromohydroquinone, and its IUPAC name is 2-bromobenzene-1,4-diol. With the molecular formula of C6H5BrO2, its product categories are Anthraquinones, Hydroquinones and Quinones; Organic Building Blocks; Oxygen Compounds; Polyols. The CAS registry number of this chemical is 583-69-7. Additionally, its classification code is Reproductive Effect. It's used in chemical reagent, water, dry powder, dry sand, carbon dioxide, foam, fire extinguishing agent 1211. Moreover, it should be stored in the dry place where the temperature is low.
Other characteristics of the 2-Bromohydroquinone can be summarised as followings: (1)ACD/LogP: 2.01; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.01; (4)ACD/LogD (pH 7.4): 2; (5)ACD/BCF (pH 5.5): 19.8; (6)ACD/BCF (pH 7.4): 19.37; (7)ACD/KOC (pH 5.5): 294.97; (8)ACD/KOC (pH 7.4): 288.48; (9)#H bond acceptors: 2; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 2; (12)Polar Surface Area: 18.46 Å2; (13)Index of Refraction: 1.657; (14)Molar Refractivity: 37.7 cm3; (15)Molar Volume: 102.4 cm3; (16)Polarizability: 14.94×10-24cm3; (17)Surface Tension: 62.2 dyne/cm; (18)Density: 1.844 g/cm3; (19)Flash Point: 122.1 °C; (20)Enthalpy of Vaporization: 53.77 kJ/mol; (21)Boiling Point: 278.3 °C at 760 mmHg; (22)Vapour Pressure: 0.00255 mmHg at 25°C.
Production method of this chemical: The 2-Bromohydroquinone could be obtained by the reactant of 1,4-diacetoxy-2-bromo-benzene. This reaction needs the reagent of 2.5 N aq. NaOH. The yield is 90 %. In addition, this reaction should be taken for 12 hours at ambient temperature.
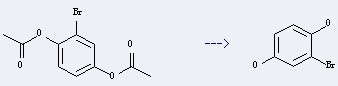
Uses of this chemical: The 2-Bromohydroquinone could react with isopropenylbenzene, and obtain the 2-methyl-2-phenyl-2,3-dihydro-benzofuran-5-ol. This reaction needs the reagents of Na2CO3, Bu4NCl, Pd(OAc)2 and H2SO4. It also needs the solvents of dimethylformamide and formic acid. The yield is 62 %. In addition, this reaction should be taken for 5 hours at the temperature of 100 °C. The other condition is heating.
When you are using this chemical, please be cautious about it as the following: This chemical is irritating to eyes, respiratory system and skin. You should wear suitable protective clothing if you use it. In case of contacting with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
1.SMILES: Brc1cc(O)ccc1O
2.InChI: InChI=1/C6H5BrO2/c7-5-3-4(8)1-2-6(5)9/h1-3,8-9H
3.InChIKey: REFDOIWRJDGBHY-UHFFFAOYAV
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View