-
Name
2-(BUTYLAMINO)ETHANOL
- EINECS 203-904-5
- CAS No. 111-75-1
- Article Data17
- CAS DataBase
- Density 0.875 g/cm3
- Solubility 1000g/L at 20℃
- Melting Point 28.94°C (estimate)
- Formula C6H15NO
- Boiling Point 199.1 °C at 760 mmHg
- Molecular Weight 117.191
- Flash Point 79.3 °C
- Transport Information UN 2735 8/PG 2
- Appearance
- Safety 26-36/37/39-45
- Risk Codes 22-34
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms 2-(Butylamino)ethanol;2-(N-Monobutylamino)ethanol;2-(n-Butylamino)ethanol;2-Hydroxyethyl(butyl)amine;Butyl(2-hydroxyethyl)amine;Butylethanolamine;MBM;N-Butyl-N-(2-hydroxyethyl)amine;N-Butylethanolamine;NSC 1098;Synergex;
- PSA 32.26000
- LogP 0.75930
Synthetic route

| Conditions | Yield |
|---|---|
| In ethanol; water at 30 - 40℃; | 78% |
| With water |
-
-
1630-71-3
2-isobutyl-3-butyloxazolidine

-
A
-
123-51-3
i-Amyl alcohol

-
B
-
111-75-1
2-butylamino-ethanol

-
C
-
91322-90-6
1-butyl-3-isopropylpyrrole

| Conditions | Yield |
|---|---|
| With potassium hydroxide Reflux; | A n/a B n/a C 73% |

-
-
51370-34-4
4-Butyl-1-oxa-4-aza-spiro[4.5]decane

-
A
-
51265-35-1
1-butyl-4,5,6,7-tetrahydro-1H-indole

-
B
-
111-75-1
2-butylamino-ethanol

-
C
-
108-93-0
cyclohexanol

| Conditions | Yield |
|---|---|
| With potassium hydroxide Heating; CH3ONa as reagent; | A 62% B n/a C n/a |


| Conditions | Yield |
|---|---|
| at 50 - 60℃; | |
| at 50 - 60℃; |

| Conditions | Yield |
|---|---|
| With ethanol; platinum Hydrogenation; |

-
A
-
1120-85-0
N-butylaziridine

-
B
-
111-75-1
2-butylamino-ethanol

-
C
-
75-07-0
acetaldehyde

-
D
-
109-73-9
N-butylamine

| Conditions | Yield |
|---|---|
| With water at 25℃; Rate constant; Mechanism; pH 10.75 (lysine buffer); H/D isotope effect; |


| Conditions | Yield |
|---|---|
| Hydrogenation; |


-
-
111-75-1
2-butylamino-ethanol

| Conditions | Yield |
|---|---|
| With sodium nitrite und Zersetzung des Reaktionsprodukts mit konz.NaOH; | |
| With sodium nitrite und Zersetzung des Reaktionsprodukts mit konz.NaOH; |
-
-
102-79-4
n-butyldiethanolamine

-
-
7664-41-7
ammonia

-
A
-
5610-49-1
N-butylpiperazine

-
B
-
111-75-1
2-butylamino-ethanol

-
C
-
30935-67-2
N-(2-aminoethyl)-N-butyl-1,2-ethanediamine

| Conditions | Yield |
|---|---|
| at 200℃; under 51485.6 Torr; |


| Conditions | Yield |
|---|---|
| With mesoporous Cs-B-Zr mixed oxide In neat (no solvent) at 220℃; under 32253.2 Torr; for 0.5h; Autoclave; Inert atmosphere; Green chemistry; |

-
-
111-75-1
2-butylamino-ethanol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate |

| Conditions | Yield |
|---|---|
| Stage #1: 2-butylamino-ethanol With potassium carbonate In acetonitrile at 20℃; for 0.166667h; Stage #2: Methyl 4-bromobutyrate In acetonitrile Reflux; | 100% |

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 24h; | 98% |

| Conditions | Yield |
|---|---|
| In methanol at 0 - 50℃; | 97.59% |
-
-
111-75-1
2-butylamino-ethanol

-
-
107291-76-9
4-Ethyl-4-hydroxyhex-2-ynenitrile

-
-
134965-81-4
2-[Butyl-(2,2-diethyl-5-imino-2,5-dihydro-furan-3-yl)-amino]-ethanol

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; for 24h; | 95% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol at 0 - 20℃; for 5h; Inert atmosphere; Schlenk technique; | 95% |
| With potassium hydroxide In ethanol at 0 - 20℃; for 5h; Schlenk technique; Inert atmosphere; | 95% |
-
-
617-86-7
triethylsilane

-
-
111-75-1
2-butylamino-ethanol

-
-
20467-03-2
1-triethylsilyloxy-2-(butylamino)ethane

| Conditions | Yield |
|---|---|
| With nickel at 130℃; for 2h; | 93% |
| With sodium |
-
-
7681-84-7
tetrahydrofuran-2-carbaldehyde

-
-
111-75-1
2-butylamino-ethanol

-
-
78749-73-2
2-(2'-tetrahydrofuryl)-3-butyl-1,3-oxazolidine

| Conditions | Yield |
|---|---|
| 92% |
-
-
111-75-1
2-butylamino-ethanol

-
-
75-36-5
acetyl chloride

-
-
19520-95-7
1-acetoxy-2-(acetyl-butyl-amino)-ethane

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran at 20℃; for 18h; Acetylation; | 92% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 0 - 20℃; for 10h; Inert atmosphere; Schlenk technique; | 89% |


| Conditions | Yield |
|---|---|
| With potassium hydroxide at 105℃; for 2h; | 87% |

| Conditions | Yield |
|---|---|
| With dichloro bis(acetonitrile) palladium(II); copper(l) iodide; oxygen In acetonitrile for 20h; Ambient temperature; | 86% |

| Conditions | Yield |
|---|---|
| With potassium fluoride on basic alumina In acetonitrile at 20℃; for 24h; | 86% |
-
-
111-75-1
2-butylamino-ethanol

-
-
74788-58-2
(1-Isothiocyanato-2-methyl-propyl)-benzene

-
-
74787-94-3
1-Butyl-1-(2-hydroxy-ethyl)-3-(2-methyl-1-phenyl-propyl)-thiourea

| Conditions | Yield |
|---|---|
| In chloroform for 1h; Heating; | 85.7% |
-
-
111-75-1
2-butylamino-ethanol

-
-
16004-15-2
1-bromomethyl-4-iodobenzene

-
-
106790-67-4
2-[Butyl-(4-iodo-benzyl)-amino]-ethanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 80℃; for 4h; | 85% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol; water Heating; | 83% |
-
-
2243-35-8
2-acetoxyacetophenone

-
-
111-75-1
2-butylamino-ethanol

-
-
21532-12-7
4-butyl-2-phenyl-morpholine

| Conditions | Yield |
|---|---|
| With formic acid at 180℃; for 20h; | 82% |
-
-
111-75-1
2-butylamino-ethanol

-
-
1214385-51-9
5-bromo-6-oxo-1,6-dihydropyridine-2-carboxylic acid

-
-
1420476-85-2
7-bromo-2-butyl-3,4-dihydro-1H-pyrido[1,2-a]pyrazine-1,6(2H)-dione

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; N-[(dimethylamino)-3-oxo-1H-1,2,3-triazolo[4,5-b]pyridin-1-yl-methylene]-N-methylmethanaminium hexafluorophosphate In dichloromethane Reflux; | 82% |
-
-
111-75-1
2-butylamino-ethanol

-
-
105627-79-0
isoquinoline-5-sulfonyl chloride hydrochloride

-
-
116970-52-6
Isoquinoline-5-sulfonic acid butyl-(2-hydroxy-ethyl)-amide

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate 1) H2O, 2) CHCl3, RT, 1 h; | 79% |
-
-
111-75-1
2-butylamino-ethanol

-
-
77545-45-0
3’,6’-dichlorospiro[3H-2,1-benzoxathiol-3,9’-[9H]xanthene]-1,1-dioxide

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; for 8h; | 78% |

| Conditions | Yield |
|---|---|
| In water for 24h; | 77.66% |
| In water for 24h; Reflux; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide 1) 110 deg C, 2 h; 2) 100 deg C, 2 h; | 75% |

| Conditions | Yield |
|---|---|
| In chloroform for 4h; Inert atmosphere; | 75% |
-
-
111-75-1
2-butylamino-ethanol

-
-
106-96-7
propargyl bromide

-
-
13105-74-3
N-Butyl-N-(2-hydroxyethyl)-propargylamin

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane; toluene at 0 - 20℃; | 75% |

| Conditions | Yield |
|---|---|
| In water for 1h; Heating; | 73% |
-
-
111-75-1
2-butylamino-ethanol

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 130℃; for 6h; | A 71% B 24% |


| Conditions | Yield |
|---|---|
| With sodium carbonate In ethanol; water Heating; | 70% |
-
-
38533-61-8
2-chloro-6-methoxy-3-nitropyridine

-
-
111-75-1
2-butylamino-ethanol

-
-
677277-87-1
2-[butyl-(6-methoxy-3-nitro-pyridin-2-yl)amino]ethanol

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water for 2h; Heating / reflux; | 70% |
-
-
111-75-1
2-butylamino-ethanol

-
-
70-11-1
α-bromoacetophenone

-
-
126806-92-6
4-Butyl-2-phenyl-morpholin-2-ol; hydrochloride

| Conditions | Yield |
|---|---|
| In diethyl ether | 68% |
2-Butylaminoethanol Consensus Reports
2-Butylaminoethanol Specification
The 2-Butylaminoethanol with CAS registry number of 111-75-1 is also known as 2-(N-Monobutylamino)ethanol. The IUPAC name and product name are the same. Its EINECS registry number is 203-904-5. In addition, the formula is C6H15NO and the molecular weight is 117.19.
Physical properties about 2-Butylaminoethanol are: (1)ACD/LogP: 0.63; (2)ACD/LogD (pH 5.5): -2.46; (3)ACD/LogD (pH 7.4): -1.85; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 6; (11)Index of Refraction: 1.435; (12)Molar Refractivity: 34.97 cm3; (13)Molar Volume: 133.8 cm3; (14)Surface Tension: 30.8 dyne/cm; (15)Density: 0.875 g/cm3; (16)Flash Point: 79.3 °C; (17)Enthalpy of Vaporization: 50.64 kJ/mol; (18)Boiling Point: 199.1 °C at 760 mmHg; (19)Vapour Pressure: 0.0875 mmHg at 25 °C.
Preparation of 2-Butylaminoethanol: it is prepared by reaction of oxirane with butylamine. The reaction needs solvent ethanol, H2O at the temperature of 30-40 °C. The yield is about 78%.
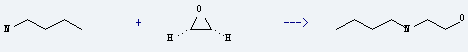
Uses of 2-Butylaminoethanol: it is used to produce 1-triethylsilyloxy-2-(butylamino)ethane by reaction with triethylsilane. The reaction occurs with reagent Ni at 130 °C for 2 hours. The yield is about 93%.
.png)
When you are using this chemical, please be cautious about it. As a chemical, it is harmful if swallowed and may cause burns. During using it, wear suitable protective clothing, gloves and eye/face protection. If contact with eyes accidently, rinse immediately with plenty of water and seek medical advice. In case of accident or if you feel unwell seek medical advice immediately.
You can still convert the following datas into molecular structure:
1. Canonical SMILES: CCCCNCCO
2. InChI: InChI=1S/C6H15NO/c1-2-3-4-7-5-6-8/h7-8H,2-6H2,1H3
3. InChIKey: LJDSTRZHPWMDPG-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mammal (species unspecified) | LD50 | oral | 7100mg/kg (7100mg/kg) | CARDIAC: CHANGE IN RATE LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION GASTROINTESTINAL: "HYPERMOTILITY, DIARRHEA" | Toxicology and Applied Pharmacology. Vol. 8, Pg. 344, 1966. |
| rat | LD50 | intraperitoneal | 840mg/kg (840mg/kg) | Toxicology and Applied Pharmacology. Vol. 12, Pg. 486, 1968. | |
| rat | LD50 | oral | 1150mg/kg (1150mg/kg) | AMA Archives of Industrial Hygiene and Occupational Medicine. Vol. 10, Pg. 61, 1954. |
Related Products
- 2-Butylaminoethanol
- 111752-26-2
- 1117-52-8
- 111755-37-4
- 1117-59-5
- 111759-96-7
- 111760-29-3
- 1117-61-9
- 111-76-2
- 111769-26-7
- 111769-27-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View