-
Name
2-Chloro-4-nitropyridine
- EINECS -0
- CAS No. 23056-36-2
- Article Data5
- CAS DataBase
- Density 1.489 g/cm3
- Solubility
- Melting Point 52-56 °C
- Formula C5H3ClN2O2
- Boiling Point 258.4 °C at 760 mmHg
- Molecular Weight 158.544
- Flash Point 110.1 °C
- Transport Information
- Appearance Light yellow powder
- Safety 26-36
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms 2-Chlor-4-nitropyridin;pyridine, 2-chloro-4-nitro-;
- PSA 58.71000
- LogP 2.16640
Synthetic route
-
-
14432-16-7
2-chloro-4-nitropyridine-N-oxide

-
-
23056-36-2
2-chloro-4-nitropyridine

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In dichloromethaneu for 4h; Reflux; | 88% |
| With phosphorus trichloride In chloroform at 20℃; Heating / reflux; | 78% |
| With ethyl acetate; phosphorus trichloride |

| Conditions | Yield |
|---|---|
| 60% |
-
-
109-09-1
2-chloropyridine

-
-
23056-36-2
2-chloro-4-nitropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: peroxyacetic acid; acetic acid 2: concentrated sulfuric acid; nitric acid 3: phosphorus (III)-chloride; ethyl acetate View Scheme |
-
-
2402-95-1
2-chloropyridine-N-oxide

-
-
23056-36-2
2-chloro-4-nitropyridine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: concentrated sulfuric acid; nitric acid 2: phosphorus (III)-chloride; ethyl acetate View Scheme |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
109-86-4
2-methoxy-ethanol

-
-
1067914-32-2
2-chloro-4-(2-methoxyethoxy)pyridine

| Conditions | Yield |
|---|---|
| With potassium tert-butylate at 0 - 20℃; for 16.5h; | 100% |
| With sodium hydride In tetrahydrofuran at 0 - 25℃; for 10h; | 98% |
| With potassium tert-butylate at 0 - 20℃; for 2h; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-Phenylimidazole With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.25h; Stage #2: 2-chloro-4-nitropyridine In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.166667h; | 100% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
7417-21-2
2-(3,4-dimethoxyphenyl)ethyl alcohol

-
-
1185999-53-4
2-chloro-4-(2-(3,4-dimethoxyphenyl)ethoxy)pyridine

| Conditions | Yield |
|---|---|
| With sodium hydride In paraffin oil (nujol); N,N-dimethyl-formamide at 0 - 20℃; for 1h; | 100% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
14704-41-7
3,5-bis(trifluoromethyl)pyrazole

| Conditions | Yield |
|---|---|
| In N-methyl-acetamide; hexane; ethyl acetate | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-nitropyridine; tributyl(1-ethoxyvinyl)stannane With bis-triphenylphosphine-palladium(II) chloride In N,N-dimethyl-formamide at 85℃; for 3h; Inert atmosphere; Stage #2: With hydrogenchloride In water at 20℃; Solvent; Temperature; Reagent/catalyst; | 99% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
14432-12-3
4-Amino-2-chloropyridine

| Conditions | Yield |
|---|---|
| With titanium for 0.25h; | 98% |
| With zinc borohydride pyridine complex In tetrahydrofuran for 2h; Heating; | 96% |
| With C36H56Cl3CrN2O; magnesium; 4,4,5,5-tetramethyl-[1,3,2]-dioxaboralane In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; chemoselective reaction; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
31846-36-3
7-hydroxy-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid

-
-
942077-61-4
7-[(2-chloropyridin-4-yl)oxy]-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-nitropyridine; 7-hydroxy-1,2,3,4-tetrahydronaphthalene-2-carboxylic acid With caesium carbonate In N,N-dimethyl-formamide at 50℃; Inert atmosphere; Stage #2: With hydrogenchloride In water at 20℃; pH=1.5; | 97% |

| Conditions | Yield |
|---|---|
| With lithium trifluoromethanesulfonate In 1-methyl-pyrrolidin-2-one at 80℃; for 12h; | 97% |
-
-
123-75-1
pyrrolidine

-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
914397-51-6
4-nitro-2-(pyrrolidin-1-yl)pyridine

| Conditions | Yield |
|---|---|
| With lithium trifluoromethanesulfonate In 1-methyl-pyrrolidin-2-one at 80℃; for 12h; | 96% |
| In tetrahydrofuran at 80℃; | 24% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
622-08-2
2-Benzyloxyethanol

-
-
1029803-69-7
4-(2-benzyloxyethoxy)-2-chloropyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-Benzyloxyethanol With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Stage #2: 2-chloro-4-nitropyridine In tetrahydrofuran at 20℃; | 96% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
111-70-6
n-heptan1ol

-
-
1069098-66-3
2-chloro-4-(heptyloxy)pyridine

| Conditions | Yield |
|---|---|
| Stage #1: n-heptan1ol With sodium hydride In tetrahydrofuran; mineral oil at 50℃; for 4h; Inert atmosphere; Stage #2: 2-chloro-4-nitropyridine In tetrahydrofuran; mineral oil at 20℃; for 24h; Inert atmosphere; | 96% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
34851-41-7
tetrabutyl ammonium methoxide

-
-
17228-69-2
2-chloro-4-methoxy-pyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; methanol at 23℃; for 2h; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
109-85-3
2-methoxyethylamine

-
-
891856-57-8
2-(2-methoxyethyl)amino-4-aminopyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-nitropyridine; 2-methoxyethylamine In ethanol for 1h; Heating / reflux; Stage #2: With hydrogen; palladium 10% on activated carbon In ethanol under 2585.81 Torr; for 2h; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
109-86-4
2-methoxy-ethanol

-
-
936112-80-0
2-(2-methoxyethoxy)-4-aminopyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-methoxy-ethanol With sodium hydride at 20℃; for 0.5h; Stage #2: 2-chloro-4-nitropyridine at 20℃; for 0.333333h; Stage #3: With hydrogen; palladium 10% on activated carbon In methanol under 2585.81 Torr; for 2h; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
1351997-30-2
((1S,4S)-4-hydroxy-1,2,3,4-tetrahydro-naphthalen-1-yl)-carbamic acid tert-butyl ester

-
-
1443243-09-1
[(1S,4S)-4-(2-chloro-pyridin-4-yloxy)-1,2,3,4-tetrahydro-naphthalen-1-yl]-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Inert atmosphere; | 95% |
| With sodium hydride In N,N-dimethyl-formamide; mineral oil at 20℃; for 0.5h; Inert atmosphere; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
6295-87-0
N-aminopyridin-1-ium iodide

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 23℃; for 10h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: diethyl malonate With sodium at 90 - 120℃; for 1.75h; Stage #2: 2-chloro-4-nitropyridine In toluene at 20 - 110℃; for 16.5h; | 95% |
| Stage #1: diethyl malonate With sodium at 90 - 120℃; for 1.75h; Stage #2: 2-chloro-4-nitropyridine In toluene at 20 - 110℃; for 16.5h; Stage #3: With hydrogenchloride; water for 3.5h; Reflux; | 95% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
141-52-6
sodium ethanolate

-
-
52311-50-9
2-chloro-4-ethoxy-pyridine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 25℃; for 12h; | 92% |
| In tetrahydrofuran at 0 - 25℃; for 12h; Large scale; | 92.4% |
| In tetrahydrofuran at 0 - 25℃; for 12h; | 92.4% |
| In tetrahydrofuran at 25℃; for 10h; | 57% |
| In tetrahydrofuran at 0 - 25℃; for 12h; |

| Conditions | Yield |
|---|---|
| With lithium trifluoromethanesulfonate In 1-methyl-pyrrolidin-2-one at 80℃; for 12h; | 92% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
75-89-8
2,2,2-trifluoroethanol

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In tetrahydrofuran at 60℃; Sealed tube; | 91% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; | 90% |
| With sodium hydride In 1,4-dioxane at 100℃; for 12h; | 52% |
| With sodium hydride In 1,4-dioxane Heating; | 52% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
945988-41-0
methyl 3-(2-chloropyridin-4-yloxy)benzoate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-hydroxybenzoate With sodium hydride In N,N-dimethyl-formamide for 0.333333h; Stage #2: 2-chloro-4-nitropyridine In N,N-dimethyl-formamide at 0 - 20℃; | 90% |
-
-
1121-25-1
2-methyl-3-pyridinol

-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
1043022-76-9
2-chloro-4-(2-methylpyridin-3-yl)oxy-pyridine

| Conditions | Yield |
|---|---|
| Stage #1: 2-methyl-3-pyridinol With sodium hydride In N,N-dimethyl-formamide for 1h; Stage #2: 2-chloro-4-nitropyridine In N,N-dimethyl-formamide at 20℃; | 90% |
-
-
110-85-0
piperazine

-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
79-03-8
propionyl chloride

-
-
947249-82-3
1-[4-(5-amino-pyridin-2-yl)-piperazin-1-yl]-propan-1-one

| Conditions | Yield |
|---|---|
| Stage #1: piperazine; 2-chloro-4-nitropyridine In ethanol for 1h; Heating / reflux; Stage #2: propionyl chloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 12h; Stage #3: With hydrogen; palladium 10% on activated carbon In ethanol under 2585.81 Torr; for 2h; | 90% |

-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
14432-16-7
2-chloro-4-nitropyridine-N-oxide

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-4-nitropyridine With urea hydrogen peroxide adduct; trifluoroacetic acid In dichloromethane at 0 - 20℃; for 72h; Stage #2: With sodium dithionite In dichloromethane; water for 0.25h; Stage #3: With hydrogenchloride In dichloromethane; water | 90% |
| With urea hydrogen peroxide adduct; trifluoroacetic anhydride In dichloromethane at 0 - 20℃; for 4.5h; | 84% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
109-89-7
diethylamine

-
-
18614-49-8
N,N-diethyl-4-nitropyridin-2-amine

| Conditions | Yield |
|---|---|
| With lithium trifluoromethanesulfonate In 1-methyl-pyrrolidin-2-one at 80℃; for 12h; | 90% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
7318-33-4
cinnamaldehyde tosylhydrazone

| Conditions | Yield |
|---|---|
| With caesium carbonate In acetonitrile at 60℃; regioselective reaction; | 90% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
1011797-06-0
N-[3-(5-amino-1-methyl-6-oxo-1,6-dihydro-pyridin-3-yl)-2-methyl-phenyl]-4-tert-butyl-benzamide

| Conditions | Yield |
|---|---|
| With caesium carbonate; tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene In 1,4-dioxane at 95℃; for 16.25h; | 85% |
-
-
23056-36-2
2-chloro-4-nitropyridine

-
-
6258-60-2
p-methoxybenzylmercaptan

-
-
945988-82-9
4-(4-methoxybenzylthio)-2-chloropyridine

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 85% |
2-Chloro-4-nitropyridine Chemical Properties
Molecular Structure of 2-Chloro-4-nitropyridine (CAS NO.23056-36-2):
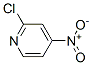
Molecular formula: C5H3ClN2O2.
Molecular Weight: 158.54
H bond acceptors: 4
H bond donors: 0
Freely Rotating Bonds: 1
Polar Surface Area: 58.71 Å2
Index of Refraction: 1.587
Molar Refractivity: 35.78 cm3
Molar Volume: 106.4 cm3
Surface Tension: 57.2 dyne/cm
Density: 1.489 g/cm3
Flash Point: 110.1 °C
Enthalpy of Vaporization: 47.6 kJ/mol
Boiling Point: 258.4 °C at 760 mmHg
Vapour Pressure: 0.0222 mmHg at 25°C
Melting point: 52-56 °C
Product Categories: Pyridine; Pyridines; Pyridine Derivertives; C5Heterocyclic Building Blocks; Halogenated Heterocycles; Heterocyclic Building Blocks
2-Chloro-4-nitropyridine Safety Profile
Hazard Codes:  Xn,
Xn, Xi
Xi
Risk Statements: 20/21/22-36/37/38
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed.
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
HazardClass: Irritant
PackingGroup: III
2-Chloro-4-nitropyridine Specification
2-Chloro-4-nitropyridine , with CAS number of 23056-36-2, can be called pyridine, 2-chloro-4-nitro- ; 2-Chlor-4-nitropyridin . It is a light yellow powder, 2-Chloro-4-nitropyridine (CAS NO.23056-36-2) is used as intermediates in fields of medicine, materials of chemical industry and so on.
Related Products
- 2-Chloro-4-nitropyridine
- 2-Chloro-4-nitropyridine 1-oxide
- 23056-39-5
- 23056-40-8
- 23056-44-2
- 23056-45-3
- 23056-46-4
- 23056-47-5
- 23056-94-2
- 23057-21-8
- 2305-79-5
- 2305-85-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View