-
Name
2-Deoxy-D-ribose
- EINECS 208-573-0
- CAS No. 533-67-5
- Article Data52
- CAS DataBase
- Density 1.516 g/cm3
- Solubility soluble in water
- Melting Point 89-90 °C(lit.)
- Formula C5H10O4
- Boiling Point 379.684 °C at 760 mmHg
- Molecular Weight 134.132
- Flash Point 197.601 °C
- Transport Information
- Appearance white to off-white crystalline powder
- Safety 24/25-37/39-36-26
- Risk Codes 20/21/22-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn,  Xi
Xi
- Synonyms Thyminose;2.2-deoxy-D-ribose;(3R,4S)-3,4,5-trihydroxypentanal;2-Deoxyribose;2-Deoxy-alpha-D-ribopyranose;3,4,5-trihydroxypentanal;(3R,4R)-3,4,5-trihydroxypentanal;D-erythro-Pentose,2-deoxy-;2-Deoxy-beta-D-erythro-pentose;2-deoxy-d-ribose/thyminose;Deoxy-Ribose;2-Deoxy-D-arabinose;D-erythro-Pentose;
- PSA 69.92000
- LogP -1.55310
Synthetic route
-
-
93857-40-0
sodium 3-deoxy-D-mannonate

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| Stage #1: sodium 3-deoxy-D-mannonate With hydrogenchloride In water pH=5; Stage #2: With sodium hypochlorite; acetic acid In water at 40 - 45℃; for 2h; pH=5 - 6; | 95% |
| Stage #1: sodium 3-deoxy-D-mannonate With hydrogenchloride In water pH=5; Stage #2: With hydrogenchloride; sodium hypochlorite; acetaldehyde In water at 40 - 45℃; for 2h; pH=5 - 6; | 94% |
| Stage #1: sodium 3-deoxy-D-mannonate With hydrogenchloride In water pH=5; Stage #2: With hydrogenchloride; sodium hypochlorite; formaldehyd In water at 40 - 45℃; for 2h; pH=5 - 6; | 94% |
-
-
1518-59-8
D-arabino-3-deoxy-hexonic acid

-
-
50480-80-3
3-deoxy-D-arabino-hexono-1,4-lactone

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| Stage #1: D-arabino-3-deoxy-hexonic acid; 3-deoxy-D-arabino-hexono-1,4-lactone With sodium carbonate In water at 20℃; pH=8.8; Stage #2: With sodium hypochlorite In water for 1 - 4h; pH=4.5 - 5.0; Stage #3: With sodium sulfite In water | 93% |
-
-
87616-31-7
(S)-2-Amino-6-{3-[(E)-3-((2R,4S,5R)-4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-ylamino)-2-methyl-acryloyl]-ureido}-hexanoic acid

-
A
-
533-67-5
2-Deoxy-D-ribose

-
B
-
76945-38-5
(2S)-amino-6-(1-thyminyl)hexanoic acid

| Conditions | Yield |
|---|---|
| With pH 10.5 In water at 90℃; | A n/a B 90% |
-
-
17510-99-5
4,5,6-trihydroxy-2-oxohexanoic acid

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With cerium(IV) sulphate; sulfuric acid; palladium 10% on activated carbon In water at 37℃; | 51% |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| Stage #1: potassium 2-dehydro-3-deoxy-D-gluconate With sulfuric acid; hydrogen; palladium 10% on activated carbon In water at 50℃; Stage #2: With calcium carbonate In water Stage #3: With calcium hydroxide; carbon dioxide; dihydrogen peroxide; copper diacetate; iron(II) sulfate more than 3 stages; | 47% |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With ammonium hydroxide |

| Conditions | Yield |
|---|---|
| With sodium periodate; water und anschliessend mit wss.Ammoniak; |

| Conditions | Yield |
|---|---|
| With calcium hydroxide; water at 120℃; und Behandeln der vom Calciumhydroxid befreiten, mit Eisen(III)-sulfat und Bariumacetat versetzen Loesung des Reaktionsprodukts mit wss.Wasserstoffperoxid; |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With calcium hydroxide; water Erwaermen des Reaktionsprodukts mit Eisen(III)-sulfat,Bariumacetat und wss.Wasserstoffperoxid; |
-
-
50705-39-0
O3-(toluene-4-sulfonyl)-D-glucose

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With calcium hydroxide; water |
-
-
91338-48-6, 91338-49-7
D-erythro-[2]pentulose-(2-nitro-phenylhydrazone)

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With water; nickel Hydrogenation.Behandlung des Reaktionsprodukts mit wss.Essigsaeure und Natriumnitrit; |
-
-
36792-87-7
2-deoxy-α-D-erythro-pentofuranose

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With diclazuril In water-d2 at 43.3℃; Equilibrium constant; Rate constant; |
-
-
89534-51-0
[R-(R*,R*)]-5-Hexene-1,2,3-triol

-
A
-
5284-18-4
D-threo-2-deoxy-pentose

-
B
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With ozone In methanol at -78℃; for 0.75h; Title compound not separated from byproducts; |
-
-
36792-88-8
2-deoxy-D-ribose

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With diclazuril In water-d2 at 43.3℃; Equilibrium constant; Rate constant; |

| Conditions | Yield |
|---|---|
| With water; potassium bromide; dinitrogen monoxide Mechanism; Irradiation; G value of product; other reagent - N2/O2 instead of N2O; |
-
-
88264-38-4
4,4-dimethyl-3-<(trimethylsilyl)oxy>pentan-2-one

-
-
15186-48-8
2,3-isopropylidene-glyceraldehyde

-
A
-
5284-18-4
D-threo-2-deoxy-pentose

-
B
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; |

-
-
74310-52-4
3-((R)-2,2-Dimethyl-[1,3]dioxolan-4-yl)-3-hydroxy-propionaldehyde

-
A
-
5284-18-4
D-threo-2-deoxy-pentose

-
B
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With acetic acid for 12h; Ambient temperature; Title compound not separated from byproducts; |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With acid |
-
-
87616-32-8
(S)-5-Guanidino-2-{3-[(E)-3-((2R,4S,5R)-4-hydroxy-5-hydroxymethyl-tetrahydro-furan-2-ylamino)-2-methyl-acryloyl]-ureido}-pentanoic acid

-
A
-
533-67-5
2-Deoxy-D-ribose

-
B
-
87616-33-9
(S)-5-Guanidino-2-(5-methyl-2,4-dioxo-3,4-dihydro-2H-pyrimidin-1-yl)-pentanoic acid

| Conditions | Yield |
|---|---|
| In water Heating; |
-
-
37117-02-5
1-(β-D-erythro-3-deoxy-pentofuranosyl)-4-methoxy-1H-pyrimidin-2-one

-
A
-
533-67-5
2-Deoxy-D-ribose

-
B
-
25902-86-7
O2-methyluracil

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 32℃; Rate constant; pH dependence; |
-
-
37085-48-6
1-(2-deoxy-β-D-erythro-pentofuranosyl)-2-methoxy-5-methyl-4(1H)-pyrimidinone

-
A
-
533-67-5
2-Deoxy-D-ribose

-
B
-
25902-91-4
2-Methoxy-5-methyl-1H-pyrimidin-4-one

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 32℃; Rate constant; pH dependence; |

| Conditions | Yield |
|---|---|
| With water; potassium bromide; dinitrogen monoxide Mechanism; Irradiation; G value of product; other reagent - N2/O2 instead of N2O; |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With sulfuric acid |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| Hydrolysis.durch enzymatische Hydrolyse und anschliessende Hydrolyse mit Hilfe eines sauren und eines schwach basischen Ionenaustauschers; |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With ethanol; palladium Hydrogenation.und Behandlung des Reaktionsprodukts mit wss.-aethanol.Natronlauge und anschliessend mit wss.Schwefelsaeure; |

-
-
533-67-5
2-Deoxy-D-ribose

| Conditions | Yield |
|---|---|
| With methanol; sodium methylate Erhitzen des Reaktionsprodukts mit wss.Essigsaeure; |
-
-
67-56-1
methanol

-
-
533-67-5
2-Deoxy-D-ribose

-
-
51255-17-5, 51255-18-6, 60134-26-1, 89577-26-4, 96038-80-1, 96039-31-5, 144301-84-8, 144301-85-9
1-O-methyl-2-deoxy-D-ribofuranoside

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 100% |
| With hydrogenchloride | 100% |
| With hydrogenchloride at 0 - 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 25℃; for 0.666667h; | 99.6% |

| Conditions | Yield |
|---|---|
| In methanol Heating; | 99% |

| Conditions | Yield |
|---|---|
| In methanol for 1h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| With acetic acid In methanol at 20℃; for 1h; | 98% |

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; N-Bromosuccinimide at 0℃; for 0.25h; | 97% |
-
-
109-80-8
1.3-propanedithiol

-
-
533-67-5
2-Deoxy-D-ribose

-
-
50907-65-8
2-deoxy-D-erythro-pentose cyclic 1,3-propanediyl mercaptal

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In chloroform at 20℃; | 90% |
| With hydrogenchloride In chloroform; water at 0 - 20℃; Inert atmosphere; | 75% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
34371-14-7
2-deoxy-D-ribonolactone

| Conditions | Yield |
|---|---|
| With bromine In water at 25℃; for 120h; | 88% |
| With bromine In water at 20℃; for 120h; | 78% |
| With bromine; potassium carbonate In water at 20℃; for 8h; | 70% |
| With bromine In water at 20℃; for 120h; Sealed tube; | |
| With bromine In water at 20℃; for 120h; Sealed tube; |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
16596-41-1
N-aminopyrrolidine

| Conditions | Yield |
|---|---|
| In methanol for 1.5h; Reflux; | 88% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 85% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
79364-36-6
(2R,3S)-hex-5-ene-1,2,3-triol

| Conditions | Yield |
|---|---|
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 2-Deoxy-D-ribose In tetrahydrofuran at 0 - 40℃; for 24h; Wittig Olefination; | 85% |
| Stage #1: Methyltriphenylphosphonium bromide With potassium tert-butylate In tetrahydrofuran at 0℃; for 0.5h; Stage #2: 2-Deoxy-D-ribose In tetrahydrofuran at 35℃; for 24h; Wittig reaction; | 75% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
75-08-1
ethanethiol

-
-
115214-11-4
2-deoxy-D-erythro-pentose diethyl dithioacetal

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 0℃; for 1h; Inert atmosphere; | 83% |
| With hydrogenchloride at 20℃; for 3h; | 71% |
| With hydrogenchloride | |
| With hydrogenchloride |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
21204-67-1
methyl (triphenylphosphoranylidene)acetate

-
-
78606-78-7
methyl (E,5S,6R)-5,6,7-trihydroxyhept-2-enoate

| Conditions | Yield |
|---|---|
| benzoic acid In 1,2-dimethoxyethane for 5h; Heating; | 82% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
78096-97-6
lithium 2-deoxy-D-erythro-pentonate

| Conditions | Yield |
|---|---|
| With potassium hydroxide; lithium hydroxide; dihydrogen peroxide; magnesium oxide 1.) 2 h, RT, 2.) 18 h, 40 deg C; | 80% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
103-49-1
dibenzylamine

-
-
910658-48-9
(2R,3S)-5-(dibenzylamino)pentane-1,2,3-triol

| Conditions | Yield |
|---|---|
| Stage #1: 2-Deoxy-D-ribose; dibenzylamine In dichloromethane for 24h; Stage #2: With sodium tetrahydroborate In dichloromethane at 0 - 20℃; for 3h; | 78% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
100-51-6
benzyl alcohol

-
-
418760-06-2
(3R,4S)-6-Benzyloxy-tetrahydro-pyran-3,4-diol

| Conditions | Yield |
|---|---|
| With dimethylsilicon dichloride at 25℃; | 76% |

| Conditions | Yield |
|---|---|
| With copper(II) bis(trifluoromethanesulfonate) In ethanol; water; toluene at 70℃; for 5h; | 75% |

| Conditions | Yield |
|---|---|
| In methanol for 0.75h; Reflux; | 73% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
64869-48-3
(2R,3S)-5-aminopentane-1,2,3-triol

| Conditions | Yield |
|---|---|
| With ammonium hydroxide; ammonium acetate; sodium cyanoborohydride In ethanol Reflux; | 73% |
| With transaminase biocatalyst ATA256; isopropylamine for 48h; Reagent/catalyst; Enzymatic reaction; | 69% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
77-76-9
2,2-dimethoxy-propane

-
-
65236-75-1
3,4-O-isopropylidene-2-deoxy-d-ribose

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetone at 0℃; | 72% |
-
-
533-67-5
2-Deoxy-D-ribose

-
-
540-63-6
ethane-1,2-dithiol

-
-
43179-62-0
2-Deoxy-D-ribose Ethylene Mercaptal

| Conditions | Yield |
|---|---|
| With hydrogenchloride 1.) 5 deg C, 10 min 2.) 1 h, room temp.; | 71% |
| With hydrogenchloride |
2-Deoxy-D-ribose Specification
The CAS register number of 2-Deoxy-D-ribose is 533-67-5. It also can be called as 2-Deoxy-D-erythropentose and the IUPAC name about this chemical is 3,4,5-trihydroxypentanal. The molecular formula about this chemical is C5H10O4 and the molecular weight is 134.13. It belongs to the following product categories, such as Pharmaceutical Raw Materials; Fine Chemical & Intermediates; 13C & 2H Sugars; Riboses and 2'-Deoxyriboses; Biochemistry; Deoxysugars; Nucleosides, Nucleotides & Related Reagents; Ribose; Sugars; Dextrins、Sugar & Carbohydrates; aldehydes; Carbohydrates & Derivatives and so on.
Physical properties about 2-Deoxy-D-ribose are: (1)ACD/BCF (pH 5.5): 1; (2)ACD/BCF (pH 7.4): 1; (3)ACD/KOC (pH 5.5): 1.193; (4)ACD/KOC (pH 7.4): 1.193; (5)#H bond acceptors: 4; (6)#H bond donors: 3; (7)#Freely Rotating Bonds: 7; (8)Polar Surface Area: 77.76Å2; (9)Index of Refraction: 1.5; (10)Molar Refractivity: 29.922 cm3; (11)Molar Volume: 101.659 cm3; (12)Polarizability: 11.862x10-24cm3; (13)Surface Tension: 61.7 dyne/cm; (14)Enthalpy of Vaporization: 72.613 kJ/mol; (15)Boiling Point: 379.684 °C at 760 mmHg.
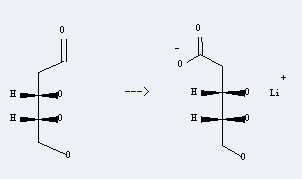
Uses of 2-Deoxy-D-ribose: it can be used to produce lithium 2-deoxy-D-erythro-pentonate at temperature of 40 °C. This reaction will need reagent 3 M KOH, 30 percent H2O2, MgO, 0.1 M LiOH with reaction time of 18 hours. The yield is about 95%.
When you are using this chemical, please be cautious about it as the following:
This chemical is harmful by inhalation, in contact with skin and if swallowed and it is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing, gloves and eye/face protection, you also need avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: C(C=O)[C@@H]([C@@H](CO)O)O
(2)InChI: InChI=1/C5H10O4/c6-2-1-4(8)5(9)3-7/h2,4-5,7-9H,1,3H2/t4-,5+/m0/s1
(3)InChIKey: ASJSAQIRZKANQN-CRCLSJGQBK
(4)Std. InChI: InChI=1S/C5H10O4/c6-2-1-4(8)5(9)3-7/h2,4-5,7-9H,1,3H2/t4-,5+/m0/s1
(5)Std. InChIKey: ASJSAQIRZKANQN-CRCLSJGQSA-N
Related Products
- 2-Deoxy-D-ribose
- 533-68-6
- 5336-87-8
- 5336-90-3
- 53369-17-8
- 533-70-0
- 5337-03-1
- 53370-51-7
- 53370-52-8
- 53370-87-9
- 53370-90-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View