-
Name
2-Heptanone
- EINECS 203-767-1
- CAS No. 110-43-0
- Article Data472
- CAS DataBase
- Density 0.808 g/cm3
- Solubility 4.3 g/L (20 °C) in water
- Melting Point -35 °C
- Formula C7H14O
- Boiling Point 151.2 °C at 760 mmHg
- Molecular Weight 114.188
- Flash Point 47.2 °C
- Transport Information UN 2810 6.1/PG 3
- Appearance colorless liquid
- Safety 36-24/25
- Risk Codes 22-38-40-48-20/22-10
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms 1-Methylhexanal;2-Oxoheptane;Amyl methyl ketone;Butylacetone;Methyl amyl ketone;Methyln-amyl ketone;Methyl n-pentyl ketone;Methyl pentyl ketone;NSC 7313;Pentylmethyl ketone;n-Amyl methyl ketone;n-Pentyl methyl ketone;
- PSA 17.07000
- LogP 2.15570
Synthetic route
-
-
52390-72-4, 543-49-7
2-Heptanol

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; [O-Cu4(triethanolamine)4(BOH)4][BF4]2 In acetonitrile at 69.84℃; for 7h; | 100% |
| With dihydrogen peroxide; bromine In dichloromethane; water at 20℃; for 2h; | 99% |
| With pyridine In dichloromethane at 20℃; for 5h; | 96% |
-
-
133619-36-0
C7H14OS

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| Ambient temperature; | 100% |
| Ambient temperature; Yield given; |
-
-
81979-61-5
2-Methyl-2-pentyl-1,3-dithiolane

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With t-butyl bromide; dimethyl sulfoxide at 70 - 75℃; for 4h; | 100% |
| With trimethylsilyl bromide; dimethyl sulfoxide In tetrachloromethane at 75 - 80℃; for 24h; | 95% |
| With thionyl chloride; dihydrogen peroxide In acetonitrile at 25℃; for 0.0333333h; | 92% |

| Conditions | Yield |
|---|---|
| With Pd(II)(15-crown-5-phen)Cl2; dinitrogen monoxide In N,N-dimethyl acetamide; water at 150℃; under 2250.23 Torr; for 18h; | 99% |
| With 2(p-CH2-C6H4CN)2[(CH2)3SO3Na]2-calix[4]arene*PdCl2; oxygen; copper dichloride under 3750.3 Torr; for 2h; Wacker oxidation; | 89% |
| With dihydrogen peroxide In water; acetonitrile at 55℃; for 12h; Wacker Oxidation; | 77% |

| Conditions | Yield |
|---|---|
| With silica gel; iron(III) chloride at 20℃; for 0.0833333h; | 98% |
| With pyridinium chlorochromate at 45℃; for 2.5h; | 91% |
| With thiourea In ethanol; water for 6h; Heating; | 89% |
| With γ-picolinium chlorochromate In dichloromethane at 20℃; for 12h; | 75% |

| Conditions | Yield |
|---|---|
| With zinc dichromate trihydrate at 20℃; grinding; neat (no solvent); chemoselective reaction; | 97% |
| With 3-carboxypyridinium dichromate In acetonitrile at 20℃; for 0h; | 96% |
| With potassium permanganate; iron(II) sulfate In dichloromethane for 5h; Heating; | 86% |
| With potassium permanganate; copper(II) sulfate In dichloromethane for 24h; Heating; | 68 % Chromat. |

| Conditions | Yield |
|---|---|
| With disodium chloro[1,3-bis(2,6-diisopropyl-4-sodiumsulfonatophenyl)imidazol-2-ylidene]gold(I); water at 100℃; for 1.17h; | 96% |
| With C22H20AuN3O2P(1+)*CF3O3S(1-); water; silver trifluoromethanesulfonate; acetic acid at 100℃; for 10h; | 92% |
| With water; trans-Bu4N[Au(2,4,6-C6F5)2Cl2] In methanol for 1.5h; Product distribution; Further Variations:; Catalysts; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With [(COD)Ir(dimethylphenylphosphine)(1,3-bis(2,4,6-trimethylphenyl)imidazolin-2-ylidene)](tetrakis(3,5-bis(trifluoromethyl)phenyl)borate); hydrogen In dichloromethane at -78 - 25℃; under 760.051 Torr; for 2h; chemoselective reaction; | 95% |
| With 1% Pd/C; hydrogen |
-
-
78465-91-5
2,2'-dimethoxyheptane

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With water; Nafion-H In acetone for 0.5h; | 94% |
| With Bi(1+)*NO3(1-)=BiNO3 In dichloromethane at 20℃; for 2h; | 78% |

| Conditions | Yield |
|---|---|
| With vanadium(III) chloride In tetrahydrofuran for 8h; Ambient temperature; | 92% |
| With phosphotungstic acid; bismuth (III) nitrate pentahydrate at 40 - 45℃; for 0.333333h; | 86% |
| With tert.-butylhydroperoxide In tetrachloromethane for 18h; Heating; | 80% |

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; nitrobenzaldehyde polymer In dichloromethane for 3h; Ambient temperature; | 89% |
| With 4-nitrobenzaldehdye; trimethylsilyl trifluoromethanesulfonate In dichloromethane for 0.0833333h; Ambient temperature; | 88% |
-
-
40513-33-5
3-aminoheptan-2-one

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With potassium bisulfite; 2-nitro-phenyl hypochlorite at 65℃; for 9h; Temperature; | 89% |

| Conditions | Yield |
|---|---|
| With 1,4-dichloro-1,4-diazoniabicyclo[2,2,2]octane bischloride In water at 50℃; for 0.25h; pH=7; | 87% |
| With dihydrogen peroxide; vanadyl acetylacetonate In acetone at 20℃; for 8h; | 82% |

| Conditions | Yield |
|---|---|
| With potassium hydroxide; 18-crown-6 ether In ethanol; benzene 1.) room temperature, 16 h, 2.) reflux, 2 h; | 86% |
| at 525℃; | |
| Darstellung; |

| Conditions | Yield |
|---|---|
| With methanol; sodium chloride electrolysis; | 85% |

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With cerium(III) chloride; silica gel for 0.0666667h; Microwave irradiation; | 85% |
-
-
103322-75-4
lithium salt of 2-heptanol

-
A
-
91-01-0
1,1-Diphenylmethanol

-
B
-
110-43-0
n-pentyl methyl ketone

-
C
-
52390-72-4, 543-49-7
2-Heptanol

| Conditions | Yield |
|---|---|
| With benzophenone In tetrahydrofuran at 24℃; for 96h; | A 78% B 15% C 84% |

-
-
103322-75-4
lithium salt of 2-heptanol

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
52390-72-4, 543-49-7
2-Heptanol

| Conditions | Yield |
|---|---|
| With benzophenone In tetrahydrofuran at 24℃; Mechanism; | A 15% B 84% |
-
-
67077-39-8
trans-3-hepten-2-ol

-
-
876-27-7
4-chlorophenyl acetate

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
5609-09-6
trans 3-hepten-2-one

| Conditions | Yield |
|---|---|
| With RuCl3H(p-cymene)2; immobilized lipase from Pseudomonas cepacia; triethylamine In dichloromethane at 20 - 25℃; for 48h; Acetylation; oxidation; reduction; Enzymatic reaction; | A n/a B n/a C 83% |


| Conditions | Yield |
|---|---|
| Heating; | A 83% B n/a |

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With bis(acetylacetonato)dioxidomolybdenum(VI); dihydrogen peroxide In acetone at 20℃; for 10h; | 82% |
-
-
79754-10-2
1-Trimethylsilanyl-heptan-2-one

-
A
-
110-43-0
n-pentyl methyl ketone

-
B
-
41055-92-9
1-chloro-2-heptanone

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; triethylamine In dichloromethane 1.) 0 deg C, 2.) room temperature; Yields of byproduct given; | A n/a B 76% |
-
-
32363-96-5
1,1-dibromohept-1-ene

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With water; zinc at 275℃; for 4h; | 76% |
-
-
121826-88-8
C9H18B(1-)*Li(1+)

-
A
-
625-25-2
2-Methyl-2-heptanol

-
B
-
110-43-0
n-pentyl methyl ketone

-
C
-
693-02-7
hex-1-yne

| Conditions | Yield |
|---|---|
| With sodium hydroxide; methanesulfonic acid; dihydrogen peroxide 1.) Et2O, -78 deg C, warming to 25 deg C, 2h; Yield given. Multistep reaction; | A 8% B n/a C 73% |
| With sodium hydroxide; methanesulfonic acid; dihydrogen peroxide 1.) Et2O, -78 deg C warming to 25 deg C, 2h; Yield given. Multistep reaction; | A 8% B 9% C n/a |


| Conditions | Yield |
|---|---|
| With formic acid; dodecacarbonyl-triangulo-triruthenium for 2.5h; Heating; | A 14% B 72% |
-
-
5609-09-6, 69668-88-8, 1119-44-4
3-hepten-2-one

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With aluminum tri-bromide; cyclohexane In various solvent(s) at 20℃; for 40h; | 70% |
| With ethanol; nickel pumice stone under 4560 Torr; Hydrogenation; | |
| With NADPH-dependent curcumin/dihydrocurcumin reductase from Escherichia coli DH10B; NADPH Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With CZYE medium (Biolife) In water for 24h; Colletotrichum gloeosporoides CBS 193.32; | A 70% B n/a |
-
-
82004-65-7
2-heptyl-hypochlorite

-
A
-
931-39-5
2-ethyl-5-methyl-tetrahydro-furan

-
B
-
110-43-0
n-pentyl methyl ketone

-
C
-
52390-72-4, 543-49-7
2-Heptanol

-
D
-
82004-68-0
5-Chloro-heptan-2-ol

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; iron(II) sulfate In tetrachloromethane Ambient temperature; protected from the light; | A 7% B 5% C n/a D 68% |

-
-
1108147-56-3
C14H21NO2

-
-
110-43-0
n-pentyl methyl ketone

| Conditions | Yield |
|---|---|
| With caesium carbonate In N,N-dimethyl-formamide at 70℃; | 68% |

| Conditions | Yield |
|---|---|
| With water; palladium dichloride at 25℃; under 1300 Torr; for 22h; in microemulsion system, closed reactor; | 66% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
52390-72-4, 543-49-7
2-Heptanol

| Conditions | Yield |
|---|---|
| With sodium aluminum tetrahydride In tetrahydrofuran at 0℃; for 0.0833333h; | 100% |
| Stage #1: n-pentyl methyl ketone With sodium tetrahydroborate at 25℃; for 0.5h; Ball milling; neat (no solvent); Stage #2: With water regiospecific reaction; | 100% |
| Stage #1: n-pentyl methyl ketone With C35H55Cl2NRuS; isopropyl alcohol at 82℃; for 0.166667h; Stage #2: With potassium hydroxide at 82℃; for 1h; Reagent/catalyst; | 100% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
104-88-1
4-chlorobenzaldehyde

-
-
100765-38-6
(E)-1-(4-chlorophenyl)oct-1-en-3-one

| Conditions | Yield |
|---|---|
| Stage #1: n-pentyl methyl ketone With piperidine In ethanol at 20℃; for 2h; Stage #2: 4-chlorobenzaldehyde In ethanol for 72h; Reflux; | 100% |
| barium dihydroxide In ethanol for 1h; Heating; | 93% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
124-68-5
2-Amino-2-methyl-1-propanol

-
-
51805-99-3
2,4,4-trimethyl-2-pentyloxazolidine

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 100% |

| Conditions | Yield |
|---|---|
| With (R)-3,3'-bis(2,4,6-triisopropylphenyl)binol phosphoric acid; 2-(3,5-dimethylphenyl)indoline In 1,3,5-trimethyl-benzene at 20 - 80℃; for 72h; Molecular sieve; Inert atmosphere; enantioselective reaction; | 100% |
| With 2-Phenylbenzothiazolin; C51H59O4P In benzene at 20℃; for 48h; Molecular sieve; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 77% |

| Conditions | Yield |
|---|---|
| With 2.5% wt Pd/C; hydrogen at 160℃; for 3h; Catalytic behavior; Dean-Stark; chemoselective reaction; | 100% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
7677-24-9
trimethylsilyl cyanide

-
-
111874-59-0
2-methyl-2-(trimethylsiloxy)heptanenitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate at 20℃; for 6h; | 99% |
| With N,N-dimethyl-(2-hydroxybenzyl)amine N-oxide In diethyl ether at 23℃; for 0.5h; | 99% |
| With 1-methoxy-2-methyl-1-(trimethylsiloxy)propene at 25℃; for 50h; | 99% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
540-63-6
ethane-1,2-dithiol

-
-
81979-61-5
2-Methyl-2-pentyl-1,3-dithiolane

| Conditions | Yield |
|---|---|
| With silica gel; zirconium(IV) chloride In dichloromethane Ambient temperature; < 5 min; | 99% |
| With cobalt(II) bromide In dichloromethane for 0.5h; Ambient temperature; | 98% |
| With P-benzyltriphenylphosphonium tribromide at 20℃; for 1.5h; | 96% |
| With P-benzyltriphenylphosphonium tribromide In dichloromethane at 20℃; for 7h; | 90% |
| With phosphorus pentoxide; silica gel for 0.0666667h; | 86% |

| Conditions | Yield |
|---|---|
| With Montmorillonite KSF In benzene for 12h; Heating; | 99% |

| Conditions | Yield |
|---|---|
| With chloro(1,5-cyclooctadiene)rhodium(I) dimer; 1,2-bis(dimethylphosphanyl)ethane; bis(pinacol)diborane In toluene at 50℃; for 18h; | 99% |
| With trifluoroacetic acid; cobalt(II) bromide; zinc In acetonitrile at 20℃; for 5h; | 65% |
| With manganese; trifluoroacetic acid; zinc dibromide; FeBr2(bpy) In N,N-dimethyl-formamide at 80℃; for 5h; | 61% |
| With [2,2]bipyridinyl; tetrabutylammonium tetrafluoroborate; iron(II) bromide In N,N-dimethyl-formamide at 20℃; Electrochemical reaction; | 60% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
120-18-3
naphthalene-2-sulfonate

-
-
95-64-7
4-amino-o-xylene

-
-
78455-20-6
N-2-heptyl-3,4-xylidine

| Conditions | Yield |
|---|---|
| With hydrogen; platinum | 99% |

-
-
110-43-0
n-pentyl methyl ketone

-
-
93-03-8
(3,4-dimethoxyphenyl)methanol

-
-
39728-59-1
1-(3,4-dimethoxyphenyl)octan-3-one

| Conditions | Yield |
|---|---|
| With potassium phosphate; 5%-palladium/activated carbon In 5,5-dimethyl-1,3-cyclohexadiene at 130℃; for 24h; Temperature; Inert atmosphere; | 99% |
| With potassium phosphate In toluene at 110℃; for 24h; Inert atmosphere; | 90% |

| Conditions | Yield |
|---|---|
| titanium tetrachloride In chloroform at -10 - 30℃; for 6h; | 98% |
| With sodium sulfate; zinc(II) chloride |

| Conditions | Yield |
|---|---|
| In diethyl ether at -30 - 0℃; for 2h; | 98% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
74-90-8
hydrogen cyanide

-
-
124-38-9
carbon dioxide

-
-
65826-54-2
5-methyl-5-n-amylhydantoin

| Conditions | Yield |
|---|---|
| Stage #1: n-pentyl methyl ketone With gallium(III) triflate; ammonia In dichloromethane at -78℃; for 3h; Inert atmosphere; Stage #2: hydrogen cyanide In dichloromethane at -78 - 25℃; Inert atmosphere; Stage #3: carbon dioxide With N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 7h; Inert atmosphere; | 98% |


| Conditions | Yield |
|---|---|
| In toluene at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate at 20℃; Neat (no solvent); | 97% |
| With sodium tetrahydroborate; silica phosphoric acid In tetrahydrofuran at 20℃; for 0.2h; | 94% |
| With sodium tetrahydroborate; silica-gel-supported sulfuric acid at 20℃; for 0.0333333h; Neat (no solvent); regioselective reaction; | 93% |
| With borohydride exchange resin; triethylamine hydrochloride In ethanol for 1h; Ambient temperature; | 89% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
120-80-9
benzene-1,2-diol

-
-
4436-30-0
2-methyl-2-n-pentyl-1,3-benzodioxole

| Conditions | Yield |
|---|---|
| With Montmorillonite KSF In toluene for 12h; Heating; | 97% |
| With toluene-4-sulfonic acid In toluene for 10h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With lithium hydroxide at 140℃; for 48h; Inert atmosphere; | 97% |
| With potassium phosphate; aluminum oxyhydroxide; palladium In toluene at 110℃; for 3h; | 90% |
| With potassium phosphate tribasic trihydrate; C39H32Cl2N5PRu In tert-Amyl alcohol at 120℃; for 12h; Inert atmosphere; Schlenk technique; | 79% |

| Conditions | Yield |
|---|---|
| With Na8H[PW9O34]*7H2O In methanol at 25℃; for 6h; Knoevenagel Condensation; | 96% |
| With molybdenum hexacarbonyl at 140℃; for 5h; Inert atmosphere; | 95% |
| With ammonium acetate; acetic acid | |
| With 0.04O40PW12(3-)*0.73Mg(2+)*0.22Al(3+)*2HO(1-)*0.98H2O In water; isopropyl alcohol at 60℃; for 6h; Knoevenagel Condensation; chemoselective reaction; | 97 %Chromat. |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate; third-generation glucose-persubstituted amidoamine dendrimer In tetrahydrofuran at -80℃; | 96% |
| With sodium tetrahydroborate; D-gluconamide PAMAM dendrimer G(3)G In tetrahydrofuran at -80℃; | 96% |
| With dried cells of Geotrichum candidum; NAD; isopropyl alcohol In various solvent(s) at 30℃; for 20h; pH=7.0; Enzymatic reaction; | 89% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate at 20℃; for 0.25h; | 96% |
| With sodium tetrahydroborate at 20℃; for 0.0333333h; Neat (no solvent); grinding; | 96% |
| With sodium tetrahydroborate In tetrahydrofuran at 20℃; for 0.0833333h; | 95% |

| Conditions | Yield |
|---|---|
| With hydrogen; K-10 montmorillonite; platinum In diethylene glycol dimethyl ether under 37503 Torr; for 24h; Reduction; | 96% |
-
-
110-43-0
n-pentyl methyl ketone

-
-
104-94-9
4-methoxy-aniline

-
-
1040047-80-0, 1103497-11-5
N-(heptan-2-yl)-4-methoxyaniline

| Conditions | Yield |
|---|---|
| With formic acid; C39H48ClIrN2O; sodium formate In water at 50℃; for 12h; pH=4.8; | 96% |
2-Heptanone Chemical Properties
IUPAC Name: heptan-2-one
Empirical Formula: C7H14O
Molecular Weight: 114.1855
EINECS: 203-388-1
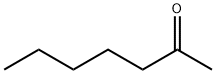
Structure of 2-Heptanone (CAS NO.110-43-0):
Index of Refraction: 1.403
Molar Refractivity: 34.5 cm3
Molar Volume: 141.2 cm3
Polarizability: 13.68×10-24cm3
Surface Tension: 24.9 dyne/cm
Density: 0.808 g/cm3
Flash Point: 47.2 °C
Enthalpy of Vaporization: 37.21 kJ/mol
Melting Point: -35 °C
Boiling Point: 151.2 °C at 760 mmHg
Vapour Pressure: 4.73 mmHg at 25°C
Storage temp: Flammables area
Water Solubility: 4.3 g/L (20 ºC)
Stability: Stable. Flammable. Incompatible with strong oxidizing agents, strong reducing agents, strong bases.
Product Categories: Industrial/Fine Chemicals;G-HAlphabetic;HA -HTAnalytical Standards;Alphabetical Listings;H;NMR Reference Standards;NMRStable Isotopes;Spectroscopy;Stable Isotopes
2-Heptanone Toxicity Data With Reference
| 1. | skn-rbt 14 mg/24H open MLD | AIHAAP American Industrial Hygiene Association Journal. 23 (1962),95. | ||
| 2. | ipr-rat LD50:800 mg/kg | 38MKAJ Pattys Industrial Hygiene and Toxicology. 2C (1982),4757. | ||
| 3. | orl-rat TDLo:45,500 mg/kg/13W-I | FCTXAV Food and Cosmetics Toxicology. 10 (1972),625. | ||
| 4. | ihl-rat LCLo:4000 ppm/4H | AIHAAP American Industrial Hygiene Association Journal. 23 (1962),95. | ||
| 5. | orl-mus LD50:730 mg/kg | APJUA8 Acta Pharmaceutica Jugoslavica. 12 (1962),79. | ||
| 6. | skn-rbt LD50:12,600 mg/kg | AIHAAP American Industrial Hygiene Association Journal. 23 (1962),95. |
2-Heptanone Consensus Reports
Reported in EPA TSCA Inventory.
2-Heptanone Safety Profile
Hazard Codes:  Xn
Xn
Risk Statements: 22-38-40-48-20/22-10
R22:Harmful if swallowed.
R38:Irritating to skin.
R40:Limited evidence of a carcinogenic effect.
R48:Danger of serious damage to health by prolonged exposure.
R20/22:Harmful by inhalation and if swallowed.
R10:Flammable.
Safety Statements: 36-24/25
S36:Wear suitable protective clothing.
S24/25:Avoid contact with skin and eyes.
RIDADR: UN 2810 6.1/PG 3
WGK Germany: 2
RTECS: MJ5250000
HazardClass: 3
PackingGroup: III
HS Code: 29141990
Moderately toxic by ingestion. Mildly toxic by inhalation and skin contact. A skin irritant. A flammable liquid when exposed to heat or flame; can react with oxidizing materials. To fight fire, use foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and fumes. See also KETONES.
2-Heptanone Standards and Recommendations
OSHA PEL: TWA 100 ppm
ACGIH TLV: TWA 50 ppm
NIOSH REL: (Ketones) TWA 465 mg/m3
DOT Classification: 3; Label: Flammable Liquid
2-Heptanone Analytical Methods
For occupational chemical analysis use NIOSH: Ketones II (Desorption in 99:1 CS2:methanol) 1301.
2-Heptanone Specification
2-Heptanone , its cas register number is 110-43-0. It also can be called Amyl methyl ketone ; Butylacetone ; Ketone C-7 ;
Ketone, methyl pentyl ; Methyl amyl ketone ; Methyl n-amyl ketone ; Methyl-amyl-cetone ; Methyl-n-amylketone ; Pentyl methyl ketone ; n-Amyl methyl ketone ; n-Pentyl methyl ketone . 2-Heptanone (CAS NO.110-43-0) is a colorless, water-white liquid with a banana-like, fruity odor.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View