-
Name
3,4-DIHYDROXY-ALLYLBENZENE
- EINECS
- CAS No. 1126-61-0
- Article Data47
- CAS DataBase
- Density 1.148 g/cm3
- Solubility
- Melting Point 42.0 to 46.0 °C
- Formula C9H10O2
- Boiling Point 289.2 °C at 760 mmHg
- Molecular Weight 150.177
- Flash Point 141.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 1,2-Benzenediol,4-(2-propenyl)- (9CI);Pyrocatechol, 4-allyl- (6CI,7CI,8CI);1,2-Dihydroxy-4-allylbenzene;1-Allyl-3,4-dihydroxybenzene;4-(2'-Propenyl)-1,2-benzenediol;4-Allyl-1,2-dihydroxybenzene;4-Allylcatechol;4-Allylpyrocatechol;Hydroxychavicol;
- PSA 40.46000
- LogP 1.82630
Synthetic route

-
-
1126-61-0
4-allylpyrocatechol

| Conditions | Yield |
|---|---|
| With water | 100% |

| Conditions | Yield |
|---|---|
| With pyridine; aluminium(III) iodide In acetonitrile at 80℃; for 18h; Reagent/catalyst; Solvent; | 99% |
| Stage #1: 4-allylguaiacol With pyridine; iodine; aluminium In acetonitrile for 18h; Reflux; Stage #2: With hydrogenchloride In water; acetonitrile at 20℃; Reagent/catalyst; | 99% |
| With aluminium(III) iodide; diisopropyl-carbodiimide In acetonitrile at 80℃; for 18h; Reagent/catalyst; | 99% |

| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; diisopropyl-carbodiimide In acetonitrile at 80℃; for 18h; | 99% |
| With aluminium(III) iodide In dimethyl sulfoxide; acetonitrile at 80℃; for 18h; | 96% |
| With aluminium(III) iodide; dimethyl sulfoxide In acetonitrile at 80℃; for 18h; | 96% |

| Conditions | Yield |
|---|---|
| With boron trichloride; tetra-(n-butyl)ammonium iodide In dichloromethane at -78℃; for 1h; dealkylation; | 88% |
| With boron trifluoride diethyl etherate In 1,4-dioxane at 20℃; for 24h; Inert atmosphere; | 55.1% |
| With boron tribromide In dichloromethane at -78 - 0℃; for 1h; | 27% |
-
-
1414854-49-1
((4-allyl-1,2-phenylene)bis(oxy))bis(triethylsilane)

-
-
1126-61-0
4-allylpyrocatechol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In tetrahydrofuran; water Inert atmosphere; | 70% |
| With hydrogenchloride In tetrahydrofuran; water at 20℃; Inert atmosphere; | 54% |

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In dichloromethane at -78℃; | 62% |
-
-
1126-20-1
2-allyloxyphenol

-
A
-
1126-61-0
4-allylpyrocatechol

-
B
-
1125-74-2
3-(2-propenyl)-1,2-benzenediol

| Conditions | Yield |
|---|---|
| at 170℃; Inert atmosphere; Neat (no solvent); | A 18% B 54% |
| at 170℃; for 2h; Inert atmosphere; | A 18% B 54% |
| In neat (no solvent) at 170℃; for 3h; Claisen Rearrangement; Inert atmosphere; Sealed tube; | A 27% B 40% |

| Conditions | Yield |
|---|---|
| With sodium carbonate; copper(l) chloride In water at 20℃; for 0.5h; | 54% |

| Conditions | Yield |
|---|---|
| With caesium carbonate In 2,2,2-trifluoroethanol for 24h; Irradiation; | 47% |

| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; N,N-dimethyl-formamide dimethyl acetal In acetonitrile at 80℃; for 18h; | 27% |
| With aluminium(III) iodide; N,N-dimethyl-formamide dimethyl acetal In acetonitrile at 80℃; for 18h; | 27% |
-
-
917-64-6
methyl magnesium iodide

-
-
93-15-2
1,2-dimethoxy-4-(2-propenyl)benzene

-
-
1126-61-0
4-allylpyrocatechol

| Conditions | Yield |
|---|---|
| With diethyl ether; xylene at 175 - 180℃; Erhitzen des vom Aether befreiten Reaktionsgemisches unter Stickstoff; |
-
-
56-23-5
tetrachloromethane

-
-
60-29-7
diethyl ether

-
-
93-15-2
1,2-dimethoxy-4-(2-propenyl)benzene

-
-
1126-61-0
4-allylpyrocatechol

| Conditions | Yield |
|---|---|
| Erhitzen des vom Aether befreiten Reaktionsgemisches auf 160-180grad; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Larrea tridentata cinnamyl alcohol acyltransferase-1 / aq. buffer / 0.5 h / 30 °C / pH 7.9 / Enzymatic reaction 2: NADH; propenylphenol synthase from Larrea tridentata / aq. buffer / 30 °C / pH 6.5 View Scheme |

-
-
1126-61-0
4-allylpyrocatechol

| Conditions | Yield |
|---|---|
| With propenylphenol synthase from Larrea tridentata; NADH In aq. buffer at 30℃; pH=6.5; Kinetics; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetone / 1 h / 20 °C 1.2: 5.5 h / 20 - 70 °C 2.1: 2 h / 170 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With aluminium(III) iodide; dimethyl sulfoxide In acetonitrile at 80℃; for 18h; Reagent/catalyst; | |
| With aluminium(III) iodide; N,N-dimethyl-formamide In acetonitrile at 80℃; for 18h; Reagent/catalyst; |
-
-
120-80-9
benzene-1,2-diol

-
A
-
1126-61-0
4-allylpyrocatechol

-
B
-
1125-74-2
3-(2-propenyl)-1,2-benzenediol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium carbonate / acetone / 0.5 h / 20 °C 1.2: 3.5 h / Reflux 2.1: neat (no solvent) / 3 h / 170 °C / Inert atmosphere; Sealed tube View Scheme |

| Conditions | Yield |
|---|---|
| With tris(pentafluorophenyl)borate |

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol at 20℃; for 1h; Inert atmosphere; | 100% |
| With hydrogen; palladium 10% on activated carbon In methanol at 20℃; for 2h; | 95% |
| With methanol; palladium 10% on activated carbon at 20℃; for 2h; | 95% |
| With triphenyl phosphite; hydrogen In chloroform at 25℃; Rate constant; Mechanism; | |
| With hydrogen; palladium on activated charcoal In ethanol; trifluoroacetic acid for 24h; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 2h; | 97% |
| With dmap; triethylamine In dichloromethane at 0 - 20℃; Inert atmosphere; | 89% |
| With pyridine | |
| With triethylamine In dichloromethane |
-
-
1126-61-0
4-allylpyrocatechol

-
-
100-39-0
benzyl bromide

-
-
857579-67-0
3-[3,4-bis(benzyloxy)phenyl]prop-1-ene

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2.5h; Inert atmosphere; | 93% |

| Conditions | Yield |
|---|---|
| Stage #1: 4-allylpyrocatechol With carbonate salt In acetone Inert atmosphere; Stage #2: propargyl bromide In acetone Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| Stage #1: d(4)-methanol With di-isopropyl azodicarboxylate; triphenylphosphine In tetrahydrofuran at 0℃; for 0.0833333h; Stage #2: 4-allylpyrocatechol In tetrahydrofuran at 0 - 20℃; for 0.5h; Mitsunobu Displacement; Inert atmosphere; | 86% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
18162-48-6
tert-butyldimethylsilyl chloride

-
-
854737-59-0
((4-allyl-1,2-phenylene)bis(oxy))bis(tert-butyldimethylsilane)

| Conditions | Yield |
|---|---|
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 0 - 20℃; for 4h; Inert atmosphere; | 81% |
| With 1H-imidazole; dmap In N,N-dimethyl-formamide at 0 - 25℃; for 16h; Inert atmosphere; | 75% |
| With 1H-imidazole; dmap In dichloromethane at 0 - 20℃; | 63% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
37381-57-0
santarubin B

| Conditions | Yield |
|---|---|
| With air In methanol; acetonitrile at 80℃; for 10h; | 74% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
458-35-5, 32811-40-8, 69056-21-9
coniferal alcohol

-
-
1309608-05-6
C19H20O5

| Conditions | Yield |
|---|---|
| With silver(l) oxide In acetone; benzene for 24h; hetero-Diels-Alder reaction; | 73% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
96-32-2
bromoacetic acid methyl ester

-
-
362467-76-3
dimethyl 2,2'-[(4-allyl-1,2-phenylene)-bis(oxy)]diacetate

| Conditions | Yield |
|---|---|
| With sodium methylate In methanol for 16h; Williamson reaction; Heating; | 65% |

| Conditions | Yield |
|---|---|
| 61.3% | |
| With potassium carbonate; sodium iodide In acetone for 4h; Heating; |
-
-
1126-61-0
4-allylpyrocatechol

-
-
458-35-5
coniferol

-
-
74741-32-5
6-allyl-3-(4-hydroxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxan

| Conditions | Yield |
|---|---|
| In acetone; benzene for 20h; Ambient temperature; | 50% |

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In methanol; hexane; acetonitrile at 0 - 20℃; | 46% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
46118-02-9
3-(3',4'-dihydroxyphenyl)-1-propanol

| Conditions | Yield |
|---|---|
| Stage #1: 4-allylpyrocatechol With dimethylsulfide borane complex In tetrahydrofuran at -10 - 20℃; for 1.25h; Inert atmosphere; Stage #2: With sodium perborate hexahydrate In tetrahydrofuran; water at 20℃; for 2h; | 35.1% |
-
-
1126-61-0
4-allylpyrocatechol

-
-
106-95-6
allyl bromide

-
A
-
1234378-77-8
4-allyl-5-propylbenzene-1,2-diol

-
B
-
1234378-87-0
3-allyl-4-propylbenzene-1,2-diol

| Conditions | Yield |
|---|---|
| Stage #1: 4-allylpyrocatechol With potassium carbonate In acetone for 1.5h; Stage #2: allyl bromide In acetone Reflux; | A 15% B 20.58% |


| Conditions | Yield |
|---|---|
| With potassium carbonate; acetone |
-
-
1126-61-0
4-allylpyrocatechol
-
-
1126-61-0
4-allylpyrocatechol

-
-
100-44-7
benzyl chloride

-
-
857579-67-0
3-[3,4-bis(benzyloxy)phenyl]prop-1-ene

| Conditions | Yield |
|---|---|
| With potassium carbonate; acetone |

| Conditions | Yield |
|---|---|
| With alkali |
-
-
1126-61-0
4-allylpyrocatechol

-
-
79-11-8
chloroacetic acid

-
-
99865-65-3
(5-allyl-2-hydroxy-phenoxy)-acetic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide |
2-Hydroxychavicol Specification
The 2-Hydroxychavicol, with the CAS registry number 1126-61-0, is also known as 1,2-Benzenediol, 4-(2-propenyl)-. This chemical's molecular formula is C9H10O2 and molecular weight is 150.1745. Its systematic name is called 4-(prop-2-en-1-yl)benzene-1,2-diol.
Physical properties of 2-Hydroxychavicol: (1)ACD/LogP: 1.90; (2)ACD/LogD (pH 5.5): 1.9; (3)ACD/LogD (pH 7.4): 1.9; (4)ACD/BCF (pH 5.5): 16.3; (5)ACD/BCF (pH 7.4): 16.21; (6)ACD/KOC (pH 5.5): 256.64; (7)ACD/KOC (pH 7.4): 255.22; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 4; (11)Index of Refraction: 1.586; (12)Molar Refractivity: 43.92 cm3; (13)Molar Volume: 130.7 cm3; (14)Surface Tension: 47.2 dyne/cm; (15)Density: 1.148 g/cm3; (16)Flash Point: 141.7 °C; (17)Enthalpy of Vaporization: 54.97 kJ/mol; (18)Boiling Point: 289.2 °C at 760 mmHg; (19)Vapour Pressure: 0.00128 mmHg at 25°C.
Preparation of 2-Hydroxychavicol: this chemical can be prepared by 4-allyl-2-methoxy-phenol. This reaction will need reagents sodium iodide, silicon tetrachloride and solvents acetonitrile, toluene. The reaction time is 20 hours. The yield is about 62%.
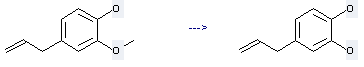
Uses of 2-Hydroxychavicol: it can be used to produce 6-allyl-3-(4-hydroxy-3-methoxyphenyl)-2-hydroxymethyl-1,4-benzodioxan at ambient temperature. This reaction will need solvents benzene and acetone with reaction time of 20 hours. The yield is about 50%.
.png)
You can still convert the following datas into molecular structure:
(1)SMILES: Oc1ccc(cc1O)C\C=C
(2)InChI: InChI=1/C9H10O2/c1-2-3-7-4-5-8(10)9(11)6-7/h2,4-6,10-11H,1,3H2
(3)InChIKey: FHEHIXJLCWUPCZ-UHFFFAOYAL
Related Products
- 2-Hydroxychavicol
- 112661-85-5
- 1126-62-1
- 112-66-3
- 112665-41-5
- 112665-43-7
- 112670-85-6
- 1126-71-2
- 112671-42-8
- 112-67-4
- 1126-74-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View