-
Name
2-Nitroaminoimidazoline
- EINECS
- CAS No. 5465-96-3
- Article Data24
- CAS DataBase
- Density 1.818 g/cm3
- Solubility
- Melting Point 219-220 °C
- Formula C3H6N4O2
- Boiling Point 255.06 °C at 760 mmHg
- Molecular Weight 130.106
- Flash Point 108.057 °C
- Transport Information
- Appearance white crystalline powder
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Imidazolidine,2-(nitroimino)- (6CI,7CI);2-Nitriminoimidazolidine;2-Nitramino-2-imidazoline;2-(Nitroimino)imidazolidine;2-(Nitroamino)-2-imidazoline;2-Nitroiminoimidazole;4,5-Dihydro-N-nitro-1H-imidazol-2-amine;
- PSA 82.24000
- LogP -0.09230
Synthetic route
-
-
141972-53-4
S,S-dimethyl N-(nitro)imidodithiocarbonate

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With ethylenediamine In chloroform | 96.1% |
-
-
107-15-3
ethylenediamine

-
-
141972-53-4
S,S-dimethyl N-(nitro)imidodithiocarbonate

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| In chloroform at 20℃; | 95% |
| In chloroform at 20 - 27℃; | 93.2% |
| In chloroform at 25 - 27℃; for 3h; | 93.2% |

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With ethylenediamine In chloroform | 92.3% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 56℃; for 8h; | 76% |
| With hydrogenchloride In water at 60℃; for 8h; Inert atmosphere; | 62% |
| With hydrogenchloride In water at 60℃; for 8h; | 61% |

| Conditions | Yield |
|---|---|
| In water at 75℃; for 1.5h; | 72% |

| Conditions | Yield |
|---|---|
| Stage #1: guanidine nitrate With sulfuric acid at 5 - 20℃; Stage #2: ethylenediamine With ammonium hydroxide at 45℃; for 12h; Temperature; | 70% |

| Conditions | Yield |
|---|---|
| Cyclization; | 58% |
| With hydrogenchloride In water |
-
-
556-88-7
nitroguanidine

-
-
333-18-6
1,2-diaminoethane dihydrochloride

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium hydroxide | 37% |
-
-
2986-25-6
methyl nitrocarbamimidothioate

-
-
7732-18-5
water

-
-
107-15-3
ethylenediamine

-
-
5465-96-3
2-(nitroimino)imidazolidine


-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 6h; Inert atmosphere; | 94% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
64-04-0
phenethylamine

-
-
74387-77-2
N'-phenethyl-2-imidazolin-2-amine

| Conditions | Yield |
|---|---|
| at 60 - 70℃; | 92% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
103669-71-2
(Z)-methyl 2-(bromomethyl)-3-phenylacrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5h; Reagent/catalyst; Inert atmosphere; | 92% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
329329-57-9
2-bromomethyl-3-p-tolyl-acrylic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5h; Inert atmosphere; | 92% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
105827-91-6
α-Cl-β-thiazolyl chloride

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Reflux; | 91.5% |
| With tetrabutylammomium bromide; potassium carbonate In toluene at 47℃; for 7h; Temperature; | 91.4% |
| With potassium carbonate In acetonitrile for 4h; Heating; | 66% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5.5h; Inert atmosphere; | 91% |

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5.5h; Inert atmosphere; | 91% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile | 90% |
| With potassium carbonate In acetonitrile | 90% |
| With potassium carbonate In acetonitrile | 90% |
| With potassium carbonate In acetonitrile | 90% |

-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
139413-75-5
methyl (2Z)-2-(bromomethyl)-3-(4-chlorophenyl)prop-2-enoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 6h; Inert atmosphere; | 90% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
88039-49-0
(Z)-ethyl 2-(bromomethyl)-3-phenylacrylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5.5h; Inert atmosphere; | 90% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
97441-93-5
N-[(4-cyano-3-methylsulfanyl-1-phenyl)pyrazol-5-yl]iminomethylenyl ethyl ether

-
-
399559-98-9
1-[(4-cyano-3-methylthio-1-phenyl)pyrazol-5-yl]iminomethyl-2-nitroiminoimidazolidine

| Conditions | Yield |
|---|---|
| With sodium hydride In acetonitrile at 20℃; for 3h; | 89% |
| sodium hydride In acetonitrile | 89.3% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-87-9
2-chloro-3-(chloromethyl)-5-methyl-6-phenylpyridine

-
-
1172115-84-2
2-{1-[(2-chloro-5-methyl-6-phenyl-3-pyridyl)methyl]-tetrahydro-1H-2-imidazolyliden}-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 89% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-89-1
ethyl-6-chloro-5-(chloromethyl)-2-phenylnicotinate

-
-
1172115-87-5
2-(1-{[2-chloro-5-(ethoxycarbonyl)-6-phenyl-3-pyridyl]-methyl}tetrahydro-1H-2-imidazolyliden)-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 88% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 6h; Inert atmosphere; | 88% |
-
-
150807-88-8
2-chloro-3-methyl-5-pyridylmethyl chloride

-
-
5465-96-3
2-(nitroimino)imidazolidine

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 20℃; for 2h; | 87% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
139413-81-3
(E)-2-(bromomethyl)-3-phenylacrylonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 5.5h; Inert atmosphere; | 87% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
139413-83-5
(E)-2-(bromomethyl)-3-(4-chlorophenyl)acrylonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile at 20℃; for 6.5h; Inert atmosphere; | 87% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
70258-18-3
2-chloro-5-(chloromethyl)pyridine

-
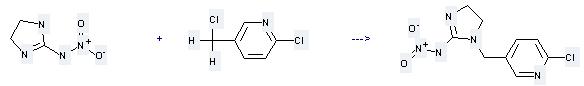
-
105827-78-9
1-(6-chloronicotinyl)-2-nitroiminoimidazoline

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Alkylation; Heating; | 86% |
| With potassium carbonate In acetonitrile Heating / reflux; | 83.5% |
| With N-benzyl-N,N,N-triethylammonium chloride; potassium carbonate In N,N-dimethyl-formamide at 35 - 40℃; | 80.5% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-81-3
2-chloro-3-(chloromethyl)-5-pentylpyridine

-
-
1205672-01-0
2-{1-[(2-chloro-5-pentyl-3-pyridyl)methyl]-tetrahydro-1H-2-imidazolyliden}-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 86% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-90-4
methyl-6-chloro-5-(chloromethyl)-2-pyridinecarboxylate

-
-
1172115-83-1
2-(1-{[2-chloro-6-(methoxycarbonyl)-3-pyridyl]-methyl}-tetrahydro-1H-2-imidazolyliden)-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile Alkylation; Heating; | 85% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
399559-99-0
1-[(4-ethoxycarbonyl-3-methylthio-1-phenyl)pyrazol-5-yl]iminomethyl-2-nitroiminoimidazolidine

| Conditions | Yield |
|---|---|
| With sodium hydride In acetonitrile at 20℃; for 3h; | 84% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-80-2
2-chloro-3-(chloromethyl)-5-isopropylpyridine

-
-
1205671-99-3
2-{1-[(2-chloro-5-isopropyl-3-pyridyl)methyl]-tetrahydro-1H-2-imidazolyliden}-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 84% |

| Conditions | Yield |
|---|---|
| With sodium hydride In N,N-dimethyl-formamide at 60℃; for 2h; Temperature; Solvent; | 83.9% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
518314-62-0
2-{1-[(2-chloro-5-methyl-3-pyridyl)methyl]-tetrahydro-1H-2-imidazolyliden}-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 82% |
-
-
5465-96-3
2-(nitroimino)imidazolidine

-
-
1205671-78-8
2-chloro-3-(chloromethyl)-5-propylpyridine

-
-
1205671-97-1
2-{1-[(2-chloro-5-propyl-3-pyridyl)methyl]-tetrahydro-1H-2-imidazolyliden}-1-oxo-1-hydraziniumolate

| Conditions | Yield |
|---|---|
| With cesium chloride; potassium carbonate In acetonitrile for 2.5h; Reflux; | 82% |
2-Nitroaminoimidazoline Specification
The 2-Nitroaminoimidazoline, with the CAS registry number 5465-96-3, is also known as N-Nitro-4,5-dihydro-1H-imidazol-2-amine. This chemical's molecular formula is C3H6N4O2 and molecular weight is 130.11. What's more, its IUPAC name is called N-(4,5-Dihydro-1H-imidazol-2-yl)nitramide.
Physical properties about 2-Nitroaminoimidazoline are: (1)ACD/LogP: -0.903; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -1.89; (4)ACD/LogD (pH 7.4): -0.98; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 6.46; (9)#H bond acceptors: 6; (10)#H bond donors: 2; (11)#Freely Rotating Bonds: 0; (12)Polar Surface Area: 82.24 Å2; (13)Index of Refraction: 1.732; (14)Molar Refractivity: 28.639 cm3; (15)Molar Volume: 71.571 cm3; (16)Polarizability: 11.353×10-24cm3; (17)Surface Tension: 89.467 dyne/cm; (18)Density: 1.818 g/cm3; (19)Flash Point: 108.057 °C; (20)Enthalpy of Vaporization: 49.252 kJ/mol; (21)Boiling Point: 255.06 °C at 760 mmHg; (22)Vapour Pressure: 0.0170 mmHg at 25 °C.
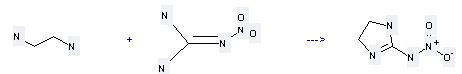
Preparation of 2-Nitroaminoimidazoline: this chemical can be prepared by ethane-1,2-diamine with nitroguanidine. This reaction needs reagent HCl at temperature of 56 °C. The reaction time is 8 hours. The yield is 76 %.
Uses of 2-Nitroaminoimidazoline: it is used to produce other chemicals. For example, it can react with 5-chloromethyl-2-chloropyridine to get 1-(6-chloronicotinyl)-2-nitroiminoimidazoline. The reaction occurs with reagent K2CO3 and solvent acetonitrile.
You can still convert the following datas into molecular structure:
(1) SMILES: [O-][N+](=O)N/C1=N/CCN1
(2) InChI: InChI=1S/C3H6N4O2/c8-7(9)6-3-4-1-2-5-3/h1-2H2,(H2,4,5,6)
(3) InChIKey: DJZWNSRUEJSEEB-UHFFFAOYSA-N
Related Products
- 2-Nitroaminoimidazoline
- 54659-85-7
- 54660-00-3
- 5466-06-8
- 54660-78-5
- 54660-80-9
- 5466-22-8
- 5466-31-9
- 546-63-4
- 54663-47-7
- 54663-78-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View