-
Name
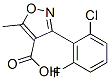
3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid
- EINECS 223-486-8
- CAS No. 3919-74-2
- Article Data13
- CAS DataBase
- Density 1.454 g/cm3
- Solubility
- Melting Point 202 °C
- Formula C11H7ClFNO3
- Boiling Point 360.7 °C at 760 mmHg
- Molecular Weight 255.633
- Flash Point 171.9 °C
- Transport Information
- Appearance
- Safety 26-36/37/39-37/39-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-(2-CHLORO-6-FLUOROPHENYL)-5-METHYLISOXAZOLE-4-CARBOXYLIC ACID;LABOTEST-BB LT00454096;RARECHEM AL BO 0550;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonitrile-4-carboxylicacid;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid 97%;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylicacid97%;3-(2-CHLORO-6-FLUOROPHENYL)-5-METHYLISOXAZOLE-4-CARBOXYLIC A;3-(2-Chloro-6-fluorophenyl)-5-methyl-4-isoxazolecarboxic Acid
- PSA 63.33000
- LogP 3.14070
Synthetic route
-
-
1239346-71-4
(3-(2-chloro-6-fluorophenyl)-5-methylisoxazol-4-yl)methanol

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: (3-(2-chloro-6-fluorophenyl)-5-methylisoxazol-4-yl)methanol With potassium permanganate; disodium hydrogenphosphate In methanol; water at 25℃; Continuous flow conditions; Sonication; Stage #2: With hydrogenchloride; sodium thiosulfate; sodium chloride In methanol; water; ethyl acetate | 87% |
-
-
51088-25-6
2-chloro-6-fluoro-N-hydroxybenzene-1-carbonimidoyl chloride

-
-
105-45-3
acetoacetic acid methyl ester

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With ethanol; triethylamine at 5℃; | 72% |
-
-
4415-09-2
methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol | |
| With water; sodium hydroxide In methanol for 1h; pH=11 - 13; Reflux; | |
| With water; sodium hydroxide In ethanol at 60℃; for 24h; | |
| With sodium hydroxide In water for 3h; |
-
-
387-45-1
2-chloro-6-fluorobenzaldehyde

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: (i) NH2OH, (ii) Cl2, CHCl3, (iii) /BRN= 506727/, MeOH 2: aq. KOH / ethanol View Scheme | |
| Multi-step reaction with 4 steps 1.1: hydroxylamine sulfate / methanol / 0.5 h 1.2: 0.5 h / pH 8 - 9 / Reflux 2.1: chlorine / methanol / 0 - 5 °C 3.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 3.2: 2 h / 0 - 20 °C / pH 9 4.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1: sodium carbonate; hydroxylamine hydrochloride / ethanol; water / 2 h / 20 °C 2: pyridine; N-chloro-succinimide / chloroform / 1.5 h / 20 °C 3: ethanol; triethylamine / 5 °C View Scheme |
-
-
51088-25-6
2-chloro-6-fluoro-N-hydroxybenzene-1-carbonimidoyl chloride

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 1.2: 2 h / 0 - 20 °C / pH 9 2.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux View Scheme |
-
-
443-33-4
N-[(2-chloro-6-fluorophenyl)methylidene]hydroxylamine

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: chlorine / methanol / 0 - 5 °C 2.1: sodium hydroxide / methanol / 0.5 h / 0 - 5 °C / pH 10 2.2: 2 h / 0 - 20 °C / pH 9 3.1: water; sodium hydroxide / methanol / 1 h / pH 11 - 13 / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1: pyridine; N-chloro-succinimide / chloroform / 1.5 h / 20 °C 2: ethanol; triethylamine / 5 °C View Scheme |
-
-
387-45-1
2-chloro-6-fluorobenzaldehyde

-
-
105-45-3
acetoacetic acid methyl ester

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 2-chloro-6-fluorobenzaldehyde With hydroxylamine sulfate In methanol at 20 - 40℃; for 1h; Stage #2: With chlorine; sodium carbonate; triethylamine In methanol at 0 - 20℃; for 24h; pH=~ 9.5; Inert atmosphere; Stage #3: acetoacetic acid methyl ester With sodium carbonate In methanol pH=~ 7 - 7.5; |
-
-
83817-51-0
ethyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In ethanol at 60℃; for 24h; |
-
-
5250-39-5, 24223-74-3
floxacillin

-
A
-
124-38-9
carbon dioxide

-
C
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium tellurite; sulfuric acid at 14.84℃; Kinetics; Thermodynamic data; Temperature; |

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With phosphorus pentachloride at 45℃; for 1h; | 88% |
| With phosphorus pentachloride In toluene | |
| With phosphorus pentachloride In toluene |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
4415-11-6
3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxamide

| Conditions | Yield |
|---|---|
| (i) SOCl2, (ii) NH3, acetone; Multistep reaction; | |
| Multi-step reaction with 2 steps 1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 1 h / Reflux 2: sodium carbonate; ammonium hydroxide / water; diethyl ether / 3.17 h / 0 - 25 °C View Scheme |
-
-
56456-50-9
(2-chloro-6-fluorophenyl)methanol

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1104461-35-9
(2-chloro-6-fluoro-phenyl)methyl N-(3-(2-chloro-6-fluoro-phenyl)-5-methyl-1,2-oxazol-4-yl)carbamate

| Conditions | Yield |
|---|---|
| With diphenyl phosphoryl azide; triethylamine In 1,4-dioxane Heating; |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-03-1
Prop-2-ynyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-19-9
(1-(2-morpholino-2-oxoethyl)-1H-1,2,3-triazol-4-yl)methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-caroboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C 3: copper(ll) sulfate pentahydrate; sodium L-ascorbate / water; tert-butyl alcohol / 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-20-2
(1-benzyl-1H-1,2,3-triazol-4-yl)methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C 3: copper(ll) sulfate pentahydrate; sodium L-ascorbate / water; tert-butyl alcohol / 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-21-3
(1-(4-(1,3-dioxoisoindolin-2-yl)butyl)-1H-1,2,3-triazol-4-yl)methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C 3: copper(ll) sulfate pentahydrate; sodium L-ascorbate / water; tert-butyl alcohol / 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-22-4
(1-butyl-1H-1,2,3-triazol-4yl)methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C 3: copper(ll) sulfate pentahydrate; sodium L-ascorbate / water; tert-butyl alcohol / 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1391026-23-5
(1-allyl-1H-1,2,3-triazol-4-yl)methyl 3-(2-chloro-6-fluorophenyl)-5-methylisoxazole-4 carboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: phosphorus pentachloride / 1 h / 45 °C 2: triethylamine / dichloromethane / 2 h / 0 - 20 °C 3: copper(ll) sulfate pentahydrate; sodium L-ascorbate / water; tert-butyl alcohol / 20 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: triethylamine / dichloromethane / 0.67 h / -5 - 0 °C 2.1: 30 - 35 °C 2.2: 1 h / -5 - 0 °C View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

-
-
1847-24-1
Flucloxacillin sodium

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: triethylamine / dichloromethane / 0.67 h / -5 - 0 °C 2.1: 30 - 35 °C 2.2: 1 h / -5 - 0 °C 3.1: sodium isooctanoate / acetone; water; ethyl acetate / 3 h / 20 - 25 °C View Scheme |
-
-
3282-30-2
pivaloyl chloride

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at -5 - 0℃; for 0.666667h; Time; |
-
-
120-78-5
di(benzothiazol-2-yl)disulfide

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| With triethylamine; triethyl phosphite In dichloromethane at 5 - 15℃; Large scale; |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 1 h / Reflux 2: sodium carbonate; ammonium hydroxide / water; diethyl ether / 3.17 h / 0 - 25 °C 3: Lawessons reagent / 1,4-dioxane / 4 h / Reflux View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: oxalyl dichloride; N,N-dimethyl-formamide / dichloromethane / 1 h / Reflux 2: sodium carbonate; ammonium hydroxide / water; diethyl ether / 3.17 h / 0 - 25 °C 3: Lawessons reagent / 1,4-dioxane / 4 h / Reflux 4: water / 24 h / Reflux View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid With thionyl chloride for 3h; Reflux; Stage #2: With hydrazine hydrate In 1,4-dioxane at 25℃; for 2h; |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: thionyl chloride / 3 h / Reflux 1.2: 2 h / 25 °C 2.1: 6 h / 140 °C / Microwave irradiation View Scheme |
-
-
2682-49-7
thiazolidine-2-one

-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid With dmap; dicyclohexyl-carbodiimide In dichloromethane at 20℃; for 0.333333h; Stage #2: thiazolidine-2-one In dichloromethane at 20℃; |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 40 °C 2: potassium carbonate / toluene / 0.42 h / 40 °C / Microwave irradiation View Scheme |
-
-
3919-74-2
3-(2-chloro-6-flourophenyl)-5-methyl-isoxazole-4-carboxylic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: thionyl chloride; N,N-dimethyl-formamide / dichloromethane / 40 °C 2: potassium carbonate / toluene / 0.42 h / 40 °C / Microwave irradiation View Scheme |
3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid Chemical Properties
CAS No:3919-74-2
Synonyms:3-(2-CHLORO-6-FLUOROPHENYL)-5-METHYLISOXAZOLE-4-CARBOXYLIC ACID;LABOTEST-BB LT00454096;RARECHEM AL BO 0550;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carbonitrile-4-carboxylicacid;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid 97%;3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylicacid97%;3-(2-CHLORO-6-FLUOROPHENYL)-5-METHYLISOXAZOLE-4-CARBOXYLIC A;3-(2-Chloro-6-fluorophenyl)-5-methyl-4-isoxazolecarboxic Acid
Molecular Formula:C11H7ClFNO3
Formula Weight:255.63
Melting Point:202 °C
Molecular Structure:
3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid Safety Profile
Risk Statements 36/37/38-37/38-36
Safety Statements 26-36/37/39-37/39-36
Hazard Note Irritant
Related Products
- 3-(2-Chloro-6-fluorophenyl)-5-methylisoxazole-4-carboxylic acid
- 3919-76-4
- 3919-81-1
- 39198-34-0
- 39198-78-2
- 39199-22-9
- 39201-27-9
- 39201-89-3
- 39202-40-9
- 3920-37-4
- 392-04-1
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View