-
Name
3-(Trifluoromethyl)benzoyl chloride
- EINECS 218-844-5
- CAS No. 2251-65-2
- Article Data79
- CAS DataBase
- Density 1.396 g/cm3
- Solubility decomposes in water
- Melting Point
- Formula C8H4ClF3O
- Boiling Point 185.5 °C at 760 mmHg
- Molecular Weight 208.567
- Flash Point 75.5 °C
- Transport Information UN 3265 8/PG 2
- Appearance clear light yellow liquid
- Safety 26-36/37/39-45-8
- Risk Codes 34-37-29
-
Molecular Structure
-
Hazard Symbols
 C
C
- Synonyms m-Toluoylchloride, a,a,a-trifluoro-(7CI,8CI);3-[Trifluoromethyl]phenylcarbonyl chloride;NSC 88307;m-(Trifluoromethyl)benzoyl chloride;
- PSA 17.07000
- LogP 3.08440
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: 3-(trifluoromethyl)benzoic acid With N,N-dimethyl-formamide In dichloromethane at 0℃; for 0.0833333h; Stage #2: With oxalyl dichloride In dichloromethane at 20℃; for 12h; | 100% |
| With thionyl chloride | |
| With thionyl chloride |

| Conditions | Yield |
|---|---|
| Stage #1: 3'-(trifluoromethyl)acetophenone With pyridine; disulfur dichloride In chlorobenzene at 20℃; for 2h; Stage #2: With sulfuryl dichloride In chlorobenzene at 20 - 132℃; for 15.5h; | 87% |
| With pyridine; disulfur dichloride at 137℃; for 20h; | 76 %Spectr. |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: carbon dioxide 2: thionyl chloride View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium hydroxide / methanol / 20 °C 1.2: pH 2 2.1: thionyl chloride / dichloromethane / 2 h / 40 °C View Scheme |
-
-
3416-93-1
2-(4,5-Dihydro-1,3-oxazol-2-yl)aniline

-
-
454-92-2
3-(trifluoromethyl)benzoic acid

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With oxalyl dichloride In dichloromethane; N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; N-chloro-succinimide; tris(2,2'-bipyridyl)ruthenium dichloride In acetonitrile at 20℃; for 24h; Sealed tube; Irradiation; |

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene In toluene at 100℃; for 12h; |
-
-
79-37-8
oxalyl dichloride

-
-
454-92-2
3-(trifluoromethyl)benzoic acid

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| In dichloromethane; N,N-dimethyl-formamide at 0 - 20℃; for 1h; |
-
-
6638-79-5
N,O-dimethylhydroxylamine*hydrochloride

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
116332-62-8
N-methoxy-N-methyl-3-trifluoromethylbenzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 0.5h; | 100% |
| With triethylamine In dichloromethane at 5℃; for 0.5h; | 98% |
| With sodium hydroxide In water at -20℃; pH=7.5 - 8; Temperature; Reagent/catalyst; Solvent; | 90% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 19h; | 100% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 19h; | 100% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 19h; | 100% |
-
-
635-22-3
4-Chloro-3-nitroaniline

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
1001341-80-5
N-(4-chloro-3-nitrophenyl)-3-(trifluoromethyl)benzamide

| Conditions | Yield |
|---|---|
| With dmap In dichloromethane at 20℃; for 6h; | 100% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
73874-95-0
(piperidin-4-yl)carbamic acid tert-butyl ester

-
-
672324-63-9
C18H23F3N2O3

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 25℃; for 3.25h; | 100% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
119-32-4
4-Methyl-3-nitroanilin

-
-
221876-21-7
N-(4-methyl-3-nitrophenyl)-3-(trifluoromethyl)benzamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-(Trifluoromethyl)benzoyl chloride; 4-Methyl-3-nitroanilin With triethylamine In dichloromethane at 25℃; for 0.333333h; Stage #2: With water In dichloromethane for 0.25h; | 100% |
| With triethylamine In dichloromethane at 25℃; for 0.333333h; | 100% |
| With triethylamine In dichloromethane at 25℃; for 0.333333h; | 100% |
-
-
99-03-6
1-(3-aminophenyl)ethanone

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
440348-37-8
N-(3-Acetyl-phenyl)-3-trifluoromethyl-benzamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane for 18h; | 100% |
| With pyridine In dichloromethane at 0 - 25℃; for 2 - 19h; Product distribution / selectivity; | 96% |
| With pyridine at 50℃; |
-
-
850451-73-9
6-(5-amino-2-chloro-phenyl)-2-methylsulfanylpyrido[2,3-d]pyrimidin-7-ylamine

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
850451-74-0
N-[3-(7-amino-2-methylsulfanyl-pyrido[2,3-d]pyrimidin-6-yl)-4-chloro-phenyl]-3-trifluoromethyl-benzamide

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 100% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
18531-95-8
(S)-2,2'-diamino-1,1'-binaphthalene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; Inert atmosphere; | 100% |
-
-
1147939-11-4
3-[6-(2-chloro-pyridin-4-yl)-thieno[2, 3-d]pyrimidin-4-yl]-phenylamine

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
1147939-12-5
N-{3-[6-(2-chloro-pyridin-4-yl)-thieno[2,3-d]pyrimidin-4-yl]-phenyl}-3-trifluoromethyl-benzamide

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; | 100% |

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 0 - 60℃; | 100% |
-
-
186593-43-1
5-amino-3-bromo-2-methylpyridine

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 2h; | 100% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 3h; |
-
-
330796-26-4
3-(4-fluoro-phenyl)-2-(1-methylamino-ethyl)-3H-quinazolin-4-one

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
330796-29-7
N-{1-[3-(4-fluorophenyl)-4-oxo-3,4-dihydroquinazolin-2-yl]ethyl}-N-methyl-3-trifluoromethylbenzamide

| Conditions | Yield |
|---|---|
| Stage #1: 3-(4-fluoro-phenyl)-2-(1-methylamino-ethyl)-3H-quinazolin-4-one; 3-(Trifluoromethyl)benzoyl chloride With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 12h; Inert atmosphere; Stage #2: With dmap In dichloromethane at 20℃; for 12h; Inert atmosphere; | 100% |

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 0 - 20℃; for 1h; | 100% |

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; Inert atmosphere; | 99.27% |
| With triethylamine In dichloromethane at 0 - 20℃; for 3h; Inert atmosphere; |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 23℃; for 1.5h; | 99% |
| With pyridine In dichloromethane at 23℃; for 24h; | 65% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With dmap In pyridine at 20℃; for 19h; | 99% |
-
-
882670-69-1
4-methyl-3-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzenamine

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
876322-58-6
N-[4-methyl-3-(4,4,5,5-tetramethyl-[1,3,2]dioxaborolan-2-yl)-phenyl]-3-trifluoromethyl-benzamide

| Conditions | Yield |
|---|---|
| In tetrahydrofuran for 2.5h; | 99% |
| In tetrahydrofuran at 0 - 20℃; for 3h; | 96% |
| In tetrahydrofuran at 0 - 20℃; for 3h; | 93% |
-
-
107-10-8
propylamine

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
53743-25-2
N-propyl-3-(trifluoromethyl)benzamide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In dichloromethane at 0 - 20℃; for 2h; Inert atmosphere; | 99% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
76786-65-7
methyl 2,3-dihydro-1H-pyrrolo[1,2-a]-pyrrole-1-carboxylate

-
-
96327-11-6
5-(3-Trifluoromethyl-benzoyl)-2,3-dihydro-1H-pyrrolizine-1-carboxylic acid methyl ester

| Conditions | Yield |
|---|---|
| In toluene for 41h; Heating; | 98% |
-
-
100227-29-0
5,6-di-O-isopropylidene-D-glucono-1,4-lactone

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
905708-57-8
5,6-di-O-isopropylidene-2,3-di-O-[3-(trifluoromethyl)benzoyl]-D-glucono-1,4-lactone

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; for 3.5h; | 98% |
-
-
950195-21-8
tert-butyl {3-[7-(3-aminophenyl)pyrazolo[1,5-a]pyrimidin-4(5H)-yl]-3-oxopropyl}carbamate

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 20℃; | 98% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
99-09-2
3-nitro-aniline

-
-
326903-68-8
N-(3-nitrophenyl)-3-(trifluoromethyl)benzamide

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; | 98% |
| With triethylamine In dichloromethane at 0 - 20℃; for 2h; | 69% |
| With triethylamine In dichloromethane at 25℃; for 15h; Inert atmosphere; Schlenk technique; | 54% |
-
-
1005781-45-2
3-(imidazo[1,2-b]pyridazin-6-yloxy)aniline

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
1005781-62-3
N-[3-(imidazo[1,2-b]pyridazin-6-yloxy)phenyl]-3-(trifluoromethyl)benzamide

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one | 98% |
| In 1-methyl-pyrrolidin-2-one at 20℃; | 98% |
-
-
109-86-4
2-methoxy-ethanol

-
-
333-20-0
potassium thioacyanate

-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| Stage #1: potassium thioacyanate; 3-(Trifluoromethyl)benzoyl chloride In acetone at 55℃; for 1h; Inert atmosphere; Stage #2: 2-methoxy-ethanol In acetone at 55℃; for 12h; | 98% |


| Conditions | Yield |
|---|---|
| With pyridine; dmap In dichloromethane at 0 - 20℃; for 2h; | 98% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

-
-
22224-41-5
1,3,5-tri-O-benzoyl-α-D-ribofuranoside

-
-
145828-13-3
α-D-1,3,5-tri-O-benzoyl-2-O-[3-(trifluoromethyl)benzoyl]ribofuranose

| Conditions | Yield |
|---|---|
| With 2,6-dimethylpyridine In dichloromethane | 97% |
| With 2,6-dimethylpyridine In dichloromethane at 20℃; for 2.5h; Acylation; | 97% |
| With pyridine In dichloromethane for 15h; 0 deg C to RT; | 89% |
| With 2,6-dimethylpyridine In dichloromethane at 0 - 20℃; Inert atmosphere; | 82% |
-
-
2251-65-2
3-(Trifluoromethyl)benzoyl chloride

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 23℃; for 36h; | 97% |
| With pyridine In dichloromethane at 23℃; for 7h; | 82% |
3-(Trifluoromethyl)benzoyl chloride Chemical Properties
Molecule structure of m-(Trifluoromethyl)benzoyl chloride (CAS NO.2251-65-2):
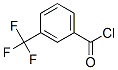
IUPAC Name: 3-(Trifluoromethyl)benzoyl chloride
Molecular Formula: C8H4ClF3O
Molecular Weight: 208.564970 g/mol
Boiling Point: 184-186 °C at 750 mm Hg(lit.)
Flash Point: 75.5 °C
Density:1.383 g/mL at 25 °C(lit.)
Index of Refraction: 1.467
Molar Refractivity: 41.47 cm3
Molar Volume: 149.3 cm3
Polarizability: 16.44×10-24 cm3
Surface Tension: 29.5 dyne/cm
Water Solubility: decomposes
Sensitive: Lachrymatory
XLogP3: 3.9
H-Bond Acceptor: 4
Rotatable Bond Count: 1
Exact Mass: 207.990277
MonoIsotopic Mass: 207.990277
Topological Polar Surface Area: 17.1
Heavy Atom Count: 13
Canonical SMILES: C1=CC(=CC(=C1)C(F)(F)F)C(=O)Cl
InChI: InChI=1S/C8H4ClF3O/c9-7(13)5-2-1-3-6(4-5)8(10,11)12/h1-4H
InChIKey: RUJYJCANMOTJMO-UHFFFAOYSA-N
EINECS: 218-844-5
Product Categories: Chemical intermediate for Flumecinol; Aromatic Halides (substituted); Benzotrifluoride Series
3-(Trifluoromethyl)benzoyl chloride Safety Profile
Hazard Codes:  C
C
Risk Statements: 34-37-29
R34:Causes burns.
R37:Irritating to respiratory system
R29:Contact with water liberates toxic gas.
Safety Statements: 26-36/37/39-45-8
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36/37/39:Wear suitable protective clothing, gloves and eye/face protection.
S45:In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible.)
S8:Keep container dry.
RIDADR: UN 3265 8/PG 2
WGK Germany: 3
F: 19-21
Hazard Note: Corrosive/Lachrymatory
TSCA: T
HazardClass: 8
PackingGroup: II
3-(Trifluoromethyl)benzoyl chloride Specification
m-(Trifluoromethyl)benzoyl chloride (CAS NO.2251-65-2) is also named as 3-(Trifluoromethyl)benzoyl chloride ; NSC 88307 ; alpha,alpha,alpha-Trifluoro-m-toluoyl chloride ; m-Toluoyl chloride, alpha,alpha,alpha-trifluoro- ; Benzoyl chloride, 3-(trifluoromethyl)- m-(Trifluoromethyl)benzoyl chloride (CAS NO.2251-65-2) is clear light yellow liquid.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View