-
Name
3,3-Dimethyl-2-oxobutyric acid
- EINECS 212-418-2
- CAS No. 815-17-8
- Article Data46
- CAS DataBase
- Density 1.09 g/cm3
- Solubility Soluble in water
- Melting Point 90.5oC
- Formula C6H10O3
- Boiling Point 182.4 °C at 760 mmHg
- Molecular Weight 130.144
- Flash Point 78.4 °C
- Transport Information
- Appearance
- Safety 26-37/39
- Risk Codes 36/37/38
-
Molecular Structure
- Hazard Symbols C
- Synonyms NSC 16648;Trimethylpyroracemic acid;Trimethylpyruvic acid;tert-Butylglyoxylic acid;Butyric acid,3,3-dimethyl-2-oxo- (6CI,7CI,8CI);Butyric acid, b,b-dimethyl-a-oxo-(4CI);2-Oxo-3,3-dimethylbutanoic acid;Trimethyl pyruvic acid;
- PSA 54.37000
- LogP 0.68620
Synthetic route

| Conditions | Yield |
|---|---|
| With ruthenium(IV) oxide; oxygen; sodium hydroxide In water at 110℃; under 30003 Torr; for 8h; pH=12; pH-value; Pressure; Reagent/catalyst; Autoclave; Inert atmosphere; | 98% |
| With bismuth(III) nitrate; sodium hydroxide; oxygen; 5%-palladium/activated carbon In water at 65 - 102.5℃; for 0.7 - 19h; | 61% |
| With sodium hydroxide; lead(II) nitrate; oxygen; 5%-palladium/activated carbon In water at 95℃; for 8h; | 5% |
-
-
1352131-48-6
5-tert-butyl-5-hydroxy-1,3-diphenyl-2,4-imidazolinedione

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide In methanol at 20 - 50℃; for 1h; | 96% |
| With methanol; sodium hydroxide at 20 - 55℃; for 2h; Temperature; | 85% |
-
-
4026-20-4
2-hydroxy-3,3-dimethylbutanoic acid

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid

| Conditions | Yield |
|---|---|
| With bismuth(III) nitrate; sodium hydroxide; oxygen; 5%-palladium/activated carbon In water at 95℃; for 2h; | A 90% B 7% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium permanganate In water at 0 - 22℃; for 3h; | 87% |
| With sodium hydroxide; potassium permanganate In water at 0℃; for 5h; | 85% |
| Stage #1: 3,3-dimethyl-butan-2-one With potassium permanganate; sodium hydroxide In water at 0 - 20℃; for 16.5h; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride In water Cooling; Inert atmosphere; Schlenk technique; | 83% |

| Conditions | Yield |
|---|---|
| triethylamine In dichloromethane at 20 - 25℃; for 3h; | A n/a B 87% |

| Conditions | Yield |
|---|---|
| triethylamine In dichloromethane at 20 - 25℃; for 60h; | A n/a B 79% |
-
-
88919-77-1
6'-(tert-butyl)spiro3.7>decane-2,3'-1',2',4'-trioxan>-5'-one

-
A
-
700-58-3
2-Adamantanone

-
B
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| triethylamine In dichloromethane at 20 - 25℃; for 16h; | A n/a B 73% |

| Conditions | Yield |
|---|---|
| triethylamine In dichloromethane at 20 - 25℃; for 16h; | A n/a B 67% |
-
-
75716-88-0
tert-butyl 3,3-dimethyl-2-oxobutanoate

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With trifluoroacetic acid at 0℃; for 1h; | 56% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; potassium permanganate; water |
-
-
855232-68-7
2-tert-butyl-4-chloro-but-1-en-3-yne

-
A
-
64-18-6
formic acid

-
B
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| durch Ozonolyse; |
-
-
2809-84-9
2-(tert-butyl)-but-1-en-3-yne

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid

| Conditions | Yield |
|---|---|
| With potassium permanganate at 0℃; |
-
-
855232-69-8
4-bromo-2-tert-butyl-but-1-en-3-yne

-
A
-
64-18-6
formic acid

-
B
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| durch Ozonolyse; |

| Conditions | Yield |
|---|---|
| With permanganate(VII) ion Oxydation; | |
| With permanganate(VII) ion |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol; water | |
| With sodium hydroxide Inert atmosphere; |
-
-
1197040-29-1
phenyl isocyanate

-
-
124-38-9
carbon dioxide

-
-
594-19-4
tert.-butyl lithium

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| (i) Me2NCH2CH2NMe2, Et2O, (ii) /BRN= 1900390/, (iii) aq. HCl; Multistep reaction; |

-
-
38559-25-0
acetic acid 3,3-dimethyl-2-oxobutyl ester

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With N-benzyl-trimethylammonium hydroxide; triphenyltetrazolium chloride In dichloromethane |
-
-
17530-24-4
di-tert-butylacetylene

-
A
-
4388-88-9
pivalil

-
B
-
1538-75-6
2,2-dimethylpropanoic anhydride

-
C
-
815-17-8
trimethylpyruvic acid

-
D
-
75-98-9
Trimethylacetic acid

| Conditions | Yield |
|---|---|
| With ozone; triphenylphosphine In chloroform-d1 at -55℃; Product distribution; other temp., other solvents, other added reagents, other concentrations; | A 0.45 mol B 0.06 mol C 0.04 mol D 0.22 mol |



-
-
815-17-8
trimethylpyruvic acid
-
-
855232-68-7
2-tert-butyl-4-chloro-but-1-en-3-yne

-
-
10028-15-6
ozone

-
A
-
64-18-6
formic acid

-
B
-
815-17-8
trimethylpyruvic acid

-
-
855232-69-8
4-bromo-2-tert-butyl-but-1-en-3-yne

-
-
10028-15-6
ozone

-
A
-
64-18-6
formic acid

-
B
-
815-17-8
trimethylpyruvic acid

-
-
105789-37-5
5-hydroxy-2,2,7,7-tetramethyl-octane-3,4,6-trione-4-oxime

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid

-
-
2809-84-9
2-(tert-butyl)-but-1-en-3-yne

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid

| Conditions | Yield |
|---|---|
| at 0℃; |

-
-
35505-57-8
2-tert-butyl-5,5-dimethyl-hexa-1,3t-diene

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid


-
-
146232-13-5
2,5,5-trimethyl-2,3-hexadienal

-
A
-
815-17-8
trimethylpyruvic acid

-
B
-
75-98-9
Trimethylacetic acid


-
-
7732-18-5
water

-
A
-
75-97-8
3,3-dimethyl-butan-2-one

-
B
-
815-17-8
trimethylpyruvic acid

-
C
-
75-98-9
Trimethylacetic acid

-
-
33105-81-6
2-amino-3,3-dimethylbutanoic acid

-
A
-
26782-71-8
D-tert-leucine

-
B
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With NADH oxidase; leucine dehydrogenase; NAD at 30℃; for 5h; pH=8.0; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 2: triphenyltetrazolium chloride, 2NMe3>OH / CH2Cl2 View Scheme |
-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With diethylzinc In tetrahydrofuran at 0 - 25℃; for 1.5h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-methoxy-pyridin-3-ylamine; trimethylpyruvic acid With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane for 24h; Stage #2: With water; ammonium chloride In 1,2-dichloro-ethane | 99% |
-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With dicyclohexyl-carbodiimide In dichloromethane at 20℃; Inert atmosphere; Glovebox; | 99% |

| Conditions | Yield |
|---|---|
| With tert-butyl methyl ether; 1,8-diazabicyclo[5.4.0]undec-7-ene at 38℃; for 23h; | 97% |
| Stage #1: trimethylpyruvic acid With 1,8-diazabicyclo[5.4.0]undec-7-ene In tert-butyl methyl ether Stage #2: ethyl bromide at 38℃; for 24h; | 97% |
| Stage #1: ethyl bromide; trimethylpyruvic acid With 1,8-diazabicyclo[5.4.0]undec-7-ene In tert-butyl methyl ether; water at 100℃; for 0.5h; Microwave heating; Stage #2: With sodium hydrogencarbonate In tert-butyl methyl ether; water | 91% |
-
-
2495-37-6
benzyl methacrylate

-
-
815-17-8
trimethylpyruvic acid

-
-
114222-35-4
benzyl 2,4,4-trimethylpentanoate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 3h; Inert atmosphere; Irradiation; | 97% |
-
-
35771-42-7
S-methylisothiocarbohydrazide

-
-
815-17-8
trimethylpyruvic acid

-
-
21087-64-9
4-amino-6-tert-butyl-3-methylthio-1,2,4-triazin-5(4H)-one

| Conditions | Yield |
|---|---|
| With sodium acetate In water at 95 - 100℃; for 3h; Reagent/catalyst; Solvent; Temperature; | 95.3% |
-
-
815-17-8
trimethylpyruvic acid

-
-
335208-46-3
2-naphthalen-1-yl-acrylic acid methyl ester

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 3h; Inert atmosphere; Irradiation; | 95% |

| Conditions | Yield |
|---|---|
| With 2-chloro-1-methyl-pyridinium iodide; triethylamine In dichloromethane for 5h; Ambient temperature; | 94% |
-
-
771581-15-8
(3-chloro-pyrazin-2-yl)-methylamine

-
-
815-17-8
trimethylpyruvic acid

-
-
1320266-96-3
C10H14ClN3O

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; silver(l) oxide In para-xylene at 80℃; for 24h; Sealed tube; Inert atmosphere; | 94% |

-
-
1007586-76-6
methyl 2-(4-tert-butylphenyl)acrylate

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 3h; Inert atmosphere; Irradiation; | 94% |

| Conditions | Yield |
|---|---|
| With palladium diacetate; 4,5-bis(diphenylphosphino)-9,9-dimethylxanthene; silver(l) oxide In para-xylene at 80℃; for 24h; Reagent/catalyst; Solvent; Sealed tube; Inert atmosphere; | 93% |

-
-
676-58-4
methylmagnesium chloride

-
-
815-17-8
trimethylpyruvic acid

-
-
4026-21-5
(R,S)-2-hydroxy-2,3,3-trimethylbutanoic acid

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; | 92% |

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In 5,5-dimethyl-1,3-cyclohexadiene at 50 - 60℃; for 3h; Reagent/catalyst; Solvent; Temperature; Inert atmosphere; | 91.3% |

| Conditions | Yield |
|---|---|
| Stage #1: trimethylpyruvic acid With sodium tetrahydroborate; sodium hydroxide In water at 0 - 20℃; for 16.5h; Inert atmosphere; Schlenk technique; Stage #2: With hydrogenchloride In water Inert atmosphere; Schlenk technique; | 91% |
| With sodium hydroxide; sodium tetrahydroborate In water at 20 - 25℃; for 24h; | 82% |
| With sodium tetrahydroborate | 77% |

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 2h; Inert atmosphere; Irradiation; | 91% |

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane | 90% |

| Conditions | Yield |
|---|---|
| With silver trifluoromethanesulfonate In water; acetonitrile at 60℃; for 24h; Schlenk technique; | 90% |
-
-
15916-56-0
(+/-)-(2-aminoethyl)phenylphosphine

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| In diethyl ether at 20℃; for 0.333333h; Inert atmosphere; Schlenk technique; | 90% |
-
-
50415-66-2
methyl 2-(4-fluorophenyl)acrylate

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 2h; Inert atmosphere; Irradiation; | 90% |
-
-
50415-59-3
methyl 2-(4-chlorophenyl)acrylate

-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 2h; Inert atmosphere; Irradiation; | 90% |
-
-
815-17-8
trimethylpyruvic acid

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; [4,4′-bis(1,1-dimethylethyl)-2,2′-bipyridine-N1,N1′]bis{3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-κN]phenyl-κC}iridium(III) hexafluorophosphate In acetone at 25℃; for 2h; Inert atmosphere; Irradiation; | 89% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene at 40 - 55℃; for 3h; Solvent; Temperature; Inert atmosphere; | 89% |

| Conditions | Yield |
|---|---|
| With pyridine; methanesulfonyl chloride In tetrahydrofuran at 0 - 20℃; for 24h; | 89% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 40℃; for 3h; pH=1.5; | 88.4% |
-
-
100-63-0
phenylhydrazine

-
-
815-17-8
trimethylpyruvic acid

-
-
38559-30-7
3,3-dimethyl-2-oxo-butanoic acid phenyhydrazone

| Conditions | Yield |
|---|---|
| In acetic acid | 87% |
| With hydrogenchloride In water at 20℃; for 2h; | 74% |
3,3-Dimethyl-2-oxobutyric acid Chemical Properties
Product Name: Trimethyl pyruvic acid (CAS NO.815-17-8)
Molecular Formula: C6H10O3
Molar mass: 130.1418g/mol
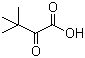
Density: 1.09 g/cm3
Appearance: needle-like crystals
Flash Point: 78.4 °C
Boiling Point: 182.4 °C at 760 mmHg
Index of Refraction: 1.439
Vapour Pressure: 0.374 mmHg at 25°C
Melting point: 90.5°C
XLogP3-AA: 1.1
H-Bond Donor: 1
H-Bond Acceptor: 3
Structure Descriptors of Trimethyl pyruvic acid (CAS NO.815-17-8):
IUPAC Name: 3,3-dimethyl-2-oxobutanoic acid
Canonical SMILES: CC(C)(C)C(=O)C(=O)O
InChI: InChI=1S/C6H10O3/c1-6(2,3)4(7)5(8)9/h1-3H3,(H,8,9)
InChIKey: IAWVHZJZHDSEOC-UHFFFAOYSA-N
3,3-Dimethyl-2-oxobutyric acid Uses
Trimethyl pyruvic acid (CAS NO.815-17-8) can be used as an intermediate of herbicide: Metribuzin.
3,3-Dimethyl-2-oxobutyric acid Production
Join dichloro-pinacolone into the reactor, and add water and a certain amount of lye, and be heated, and then dropping sodium hypochlorite solution to be oxidatized at low temperatures and the presence of catalyst.Obtained the filtrate by filtering.
3,3-Dimethyl-2-oxobutyric acid Safety Profile
Safety Information of Trimethyl pyruvic acid (CAS NO.815-17-8):
Risk Statements:
36: Irritating to the eyes
37: Irritating to the respiratory system
38: Irritating to the skin
Safety Statements:
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
37: Wear suitable gloves
39: Wear eye/face protection
3,3-Dimethyl-2-oxobutyric acid Specification
Trimethyl pyruvic acid , its CAS NO. is 815-17-8, the synonyms are tert-Butylglyoxylic acid ; 3,3-Dimethyl-2-oxobutanoic acid ; Butyric acid, 3,3-dimethyl-2-oxo- ; 3,3-Dimethyl-2-oxobutyric acid ; 3,3-Dimethyl-2-oxo-butanoic acid ; Butanoic acid, 3,3-dimethyl-2-oxo- ; Pyruvic acid, trimethyl- ; Glyoxylic acid, tert-butyl- .
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 81522-68-1
- 815-23-6
- 815-24-7
- 81525-10-2
- 81525-64-6
- 81-52-7
- 81536-18-7
- 81-53-8
- 81541-12-0
- 81542-16-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View