-
Name
3,4-Dichlorobenzaldehyde
- EINECS 228-520-5
- CAS No. 6287-38-3
- Article Data55
- CAS DataBase
- Density 1.4 g/cm3
- Solubility insoluble in water
- Melting Point 39-42 °C(lit.)
- Formula C7H4Cl2O
- Boiling Point 248.8 °C at 760 mmHg
- Molecular Weight 175.014
- Flash Point 102 °C
- Transport Information UN 1759 8/PG 2
- Appearance White crystalline solid or chunky powder
- Safety 26-27-28-36/37/39-45
- Risk Codes 34-36/37/38
-
Molecular Structure
-
Hazard Symbols
 C,
C,  Xi
Xi
- Synonyms 3,4-Dichlorobenzenecarboxaldehyde;NSC 8763;m,p-Dichlorobenzaldehyde;
- PSA 17.07000
- LogP 2.80590
Synthetic route

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide at 80℃; for 7h; Green chemistry; | 98% |
| With dmap; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; copper diacetate In neat (no solvent) at 100℃; for 18h; Green chemistry; | 97% |
| With 2C36H60N9(3+)*3O4W(2-); dihydrogen peroxide In water at 20℃; for 0.166667h; Green chemistry; | 96% |
-
-
1162648-58-9
3,4-Cl2-Ph-CH2OTMS

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With copper(II) nitrate trihydrate; water; silica gel; potassium bromide at 90℃; for 0.7h; Neat (no solvent); | 90% |

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In diethyl ether; toluene at 20℃; for 2h; Inert atmosphere; | 88% |

| Conditions | Yield |
|---|---|
| With benzo[c]cinnoline; sodium iodide In N,N-dimethyl-formamide at 80℃; Kornblum Aldehyd Synthesis; | 85% |

| Conditions | Yield |
|---|---|
| With triethylamine; palladium dichloride In water; N,N-dimethyl-formamide at 110℃; under 760.051 Torr; for 4h; Inert atmosphere; | 83% |
-
-
36480-40-7
3,4-dichlorobenzyl mercaptan

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With dipotassium peroxodisulfate; tetrakis(pyridine)silver(II) peroxodisulfate; oxygen In N,N-dimethyl-formamide at 23℃; under 760.051 Torr; Irradiation; | 65% |
-
-
163226-27-5
tert-butyl 3,4-dichlorobenzylidenecarbamate

-
A
-
6287-38-3
3,4-dichlorobenzaldehyde

-
C
-
163226-31-1
N-tert-butoxycarbonyl-3-(3,4-dichlorophenyl)oxaziridine

| Conditions | Yield |
|---|---|
| With n-butyllithium; 3-chloro-benzenecarboperoxoic acid In hexane; dichloromethane -78 degC, 2 h, room t., 30 min; | A 21 % Spectr. B 8 % Spectr. C 63% |
| With n-butyllithium; 3-chloro-benzenecarboperoxoic acid In hexane; dichloromethane -78 degC, 2 h, room t., 30 min; Title compound not separated from byproducts; | A 21 % Spectr. B 8 % Spectr. C 71 % Spectr. |

-
-
13014-24-9
3,4-dichloro-1-trichloromethylbenzene

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With 10% Pt/activated carbon; hydrogen; tri-n-butyl-tin hydride In N,N-dimethyl acetamide at 25℃; under 760.051 Torr; for 2h; chemoselective reaction; | 51% |

-
B
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With oxygen; mercury In thiophene; benzene for 0.416667h; UV-irradiation; | A 40% B n/a |

| Conditions | Yield |
|---|---|
| With sodium molybdate; dihydrogen peroxide; cobalt(II) acetate; acetic acid; sodium bromide at 90℃; for 0.25h; Temperature; | 29% |
-
-
16588-34-4
4-chloro-3-nitro-benzaldehyde

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium dithionite; water Diazotieren und Umsetzen mit Kupfer(I)-chlorid in Salzsaeure; |

| Conditions | Yield |
|---|---|
| With 2-morpholin-4-yldisulfanyl-benzothiazole |
-
-
2039-83-0
3,4-dichlorostyrene

-
A
-
42981-08-8
2,3',4'-trichloroacetophenone

-
B
-
91309-68-1
1,2-Dichloro-4-((Z)-2-chloro-vinyl)-benzene

-
C
-
91309-69-2
1,2-Dichloro-4-((E)-2-chloro-vinyl)-benzene

-
D
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With chlorine dioxide In tetrachloromethane at 52 - 54℃; for 2h; Further byproducts given. Title compound not separated from byproducts; | A 25.1 % Chromat. B 3.1 % Chromat. C 19.8 % Chromat. D 1.0 % Chromat. |


| Conditions | Yield |
|---|---|
| With trichlorophosphate In N,N-dimethyl-formamide |

| Conditions | Yield |
|---|---|
| With trichlorophosphate In N,N-dimethyl-formamide |

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid at 30 - 40℃; |

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With water; calcium carbonate |

| Conditions | Yield |
|---|---|
| anschliessenden Erhitzen mit wss.HCl; |

| Conditions | Yield |
|---|---|
| at 220℃; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Br2 2: Mor View Scheme |
-
-
1403840-46-9
(1E,4E)-1-(3,4-dichlorophenyl)-5-(2,6,6-trimethylcyclohex-1-en-1-yl)penta-1,4-dien-3-one

-
B
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: oxygen / thiophene; benzene / 0.67 h / UV-irradiation 2: oxygen; mercury / thiophene; benzene / 0.42 h / UV-irradiation View Scheme |

| Conditions | Yield |
|---|---|
| With oxygen; benzaldehyde In toluene for 2.25h; Reflux; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / 0 - 20 °C / Inert atmosphere 2: pyridinium chlorochromate / dichloromethane View Scheme |
-
-
67-64-1
acetone

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
30983-80-3
(1E,4E)-1,5-bis(3,4-dichlorophenyl)penta-1,4-dien-3-one

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; for 1h; | 100% |
| With sodium hydroxide In ethanol; water at 20℃; Aldol Addition; | 70% |
| With sodium hydroxide | |
| With sodium hydroxide In ethanol |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0℃; Inert atmosphere; | 100% |
| With potassium fluoride on basic alumina; formaldehyd for 0.0666667h; cross-Cannizzaro reaction; microwave irradiation; | 93% |
| With sodium tetrahydroborate In methanol at 0 - 20℃; for 4h; | 90% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 2h; | 100% |
| With sodium hydroxide In water at 20℃; for 2h; | 100% |
| With sodium hydroxide for 72h; Ambient temperature; | 70% |
-
-
6285-64-9
phenylazomalonamamidine hydrochloride

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
81976-28-5
2,8-Bis-(3,4-dichloro-phenyl)-1,7-dihydro-purin-6-one

| Conditions | Yield |
|---|---|
| at 170℃; for 1h; | 100% |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
22483-09-6
2,2-dimethoxyethylamine

-
-
73274-27-8
N-<(3,4-dichlorophenyl)methylene>-2,2-dimethoxyethanamine

| Conditions | Yield |
|---|---|
| In toluene for 1.5h; Heating; | 100% |
| In toluene for 0.5h; Heating; | 100% |
| for 0.333333h; Heating; |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
22483-09-6
2,2-dimethoxyethylamine

-
-
73274-27-8
[1-(3,4-Dichloro-phenyl)-meth-(Z)-ylidene]-(2,2-dimethoxy-ethyl)-amine

| Conditions | Yield |
|---|---|
| In benzene Heating; | 100% |
| In toluene Heating / reflux; |
-
-
557-20-0
diethylzinc

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
742107-59-1
(S)-1-(3,4-Dichloro-phenyl)-propan-1-ol

| Conditions | Yield |
|---|---|
| With titanium(IV) isopropylate; N,N’-(1R,2R)-cyclohexane-1,2-diylbis(1,1,1-trifluoromethanesulfonamide) In toluene at -78℃; | 100% |
| Stage #1: diethylzinc; 3,4-dichlorobenzaldehyde With titanium(IV) isopropylate; N,N’-(1R,2R)-cyclohexane-1,2-diylbis(1,1,1-trifluoromethanesulfonamide) In toluene at -78 - 20℃; Stage #2: With hydrogenchloride In water; toluene | |
| Stage #1: diethylzinc With titanium(IV) isopropylate; N,N’-(1R,2R)-cyclohexane-1,2-diylbis(1,1,1-trifluoromethanesulfonamide) In toluene at -78 - 40℃; for 0.666667h; Stage #2: 3,4-dichlorobenzaldehyde In toluene at -78 - 20℃; | |
| Stage #1: diethylzinc With titanium(IV) isopropylate; N,N’-(1R,2R)-cyclohexane-1,2-diylbis(1,1,1-trifluoromethanesulfonamide) In toluene at -78 - 40℃; for 0.666667h; Stage #2: 3,4-dichlorobenzaldehyde In toluene at 0 - 20℃; Stage #3: With hydrogenchloride; water In toluene at 20℃; |
-
-
4202-14-6
dimethyl (2-oxopropyl)phosphonate

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
55420-70-7
(E)-4-(3,4-dichlorophenyl)but-3-en-2-one

| Conditions | Yield |
|---|---|
| With potassium carbonate In water at 5℃; for 0.25h; | 100% |
-
-
107-21-1
ethylene glycol

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
773093-44-0
2-(3,4-dichlorophenyl)-1,3-dioxolane

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene for 6h; Reflux; Dean-Stark; | 100% |
| With poly(ethylene glycol) 1000 based dicationic acidic ionic liquid In toluene at 80℃; for 1h; Reagent/catalyst; Ionic liquid; | 84% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) for 0.05h; | 100% |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In acetic acid at 90℃; for 7h; | 99% |
| With dihydrogen peroxide; benzeneseleninic acid In tetrahydrofuran for 8h; Heating; | 98% |
| With oxygen; Langlois reagent In acetonitrile at 25℃; under 760.051 Torr; for 12h; Irradiation; Green chemistry; | 95% |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
389082-07-9
γ-aminopropyl-2-methyl-6-phenyl-1,3-dioxa-6-aza-2-silacyclooctane

| Conditions | Yield |
|---|---|
| With potassium carbonate In benzene at 20℃; for 2h; | 99% |
-
-
1005794-49-9
tert-butyl 1-(2-methylallyl)hydrazinecarboxylate

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; | 99% |
-
-
141-97-9
ethyl acetoacetate

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
109-77-3
malononitrile

-
-
315244-85-0, 874193-25-6
6-amino-4-(3,4-dichlorophenyl)-2,4-dihydro-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With hydrazine hydrate; heptakis(6-amino-6-deoxy)-β-cyclodextrin at 20℃; for 0.0166667h; Neat (no solvent); | 99% |


| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane; 1-(2-hydroxyethyl)pyridinium chloride In water at 20℃; for 0.416667h; Morita-Baylis-Hillman Alkylation; Green chemistry; | 99% |
| With 1,4-diaza-bicyclo[2.2.2]octane; choline chloride; glycerol In water at 20℃; for 2h; Morita-Baylis-Hillman Alkylation; Green chemistry; | 94% |
| With 1,4-diaza-bicyclo[2.2.2]octane In 1,4-dioxane; water at 20℃; for 4h; Baylis-Hillman reaction; | 68% |
-
-
72824-04-5
2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 3,4-dichlorobenzaldehyde With (R)-6,6'-bis(2,4,6-triisopropylphenyl)-1,1'-spirobiindanyl-7,7'-diyl hydrogen phosphate In toluene at -70℃; Inert atmosphere; Stage #2: 2-Allyl-4,4,5,5-tetramethyl-1,3,2-dioxaborolane In toluene at -70℃; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
39082-53-6
1,1-bis(phenylsulfonyl)ethylene

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With (S)-N-(3,5-bis(trifluoromethyl)phenyl)-2-(diphenyl ((tri-methylsilyl)oxy)methyl)pyrrolidine-1-carbothioamide; benzoic acid In dichloromethane; water at 20℃; for 18h; Michael Addition; enantioselective reaction; | A 99% B n/a |


| Conditions | Yield |
|---|---|
| Stage #1: 3,4-dichlorobenzaldehyde; N-methylaniline With silicotungstic acid supported on Amberlyst 15 beads (WSi/A15 catalyst) In neat (no solvent) at 20℃; for 0.166667h; Friedel-Crafts Alkylation; Inert atmosphere; Green chemistry; Stage #2: indole With silicotungstic acid supported on Amberlyst 15 beads (WSi/A15 catalyst) In neat (no solvent) at 20℃; for 5h; Catalytic behavior; Friedel-Crafts Alkylation; Inert atmosphere; Green chemistry; regioselective reaction; | 99% |


| Conditions | Yield |
|---|---|
| With lithium hydroxide In ethanol; water at 23 - 27℃; for 0.00277778h; Claisen-Schmidt Condensation; Sonication; | 99% |
-
-
95-54-5
1,2-diamino-benzene

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
67370-32-5
2-(3,4-dichlorophenyl)-1H-benzo[d]imidazole

| Conditions | Yield |
|---|---|
| With air In ethanol at 20℃; for 6h; Green chemistry; | 98.1% |
| With sodium disulfite In N,N-dimethyl-formamide for 2h; Reflux; | 91% |
| With ammonium acetate at 70℃; for 1h; | 74% |
-
-
98-86-2
acetophenone

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
22966-16-1
3-(3,4-dichlorophenyl)-1-phenyl-2-propen-1-one

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 100℃; for 3h; | 98% |
| With Musa sp. 'Malbhog' peel ash In neat (no solvent) at 20℃; for 0.3h; Reagent/catalyst; Claisen-Schmidt Condensation; | 86% |
| With potassium hydroxide In ethanol; water at 20℃; Claisen-Schmidt Condensation; | 30% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
95392-03-3
2-(3,4-dichlorophenyl)-2-((trimethylsilyl)oxy)acetonitrile

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 24h; | 98% |
| With zinc(II) iodide at 5 - 20℃; for 0.333333h; Yield given; | |
| With zinc(II) iodide |
-
-
6285-64-9
phenylazomalonamamidine hydrochloride

-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
81976-33-2
2-{N-[1-(3,4-Dichloro-phenyl)-meth-(E)-ylidene]-carbamimidoyl}-2-phenylazo-acetamide; hydrochloride

| Conditions | Yield |
|---|---|
| at 120℃; for 0.25h; | 98% |

| Conditions | Yield |
|---|---|
| In benzene Heating; | 98% |
| In benzene | 98% |
| In benzene | 98% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol for 12h; Heating; | 98% |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
105-53-3
diethyl malonate

-
-
40878-09-9
diethyl 2-(3,4-dichlorobenzylidene)malonate

| Conditions | Yield |
|---|---|
| piperidine; benzoic acid In benzene Condensation; refluxing overnight under an argon atmosphere with Dean-Stark trap; | 98% |
| With piperidine; acetic acid In toluene Reflux; | |
| With piperidine; acetic acid In toluene for 6h; Knoevenagel condensation; Reflux; | |
| With piperidine In 5,5-dimethyl-1,3-cyclohexadiene Reflux; Inert atmosphere; Sealed tube; | |
| Knoevenagel Condensation; Sealed tube; |
-
-
28144-70-9
anthranilic acid amide

-
-
6287-38-3
3,4-dichlorobenzaldehyde

| Conditions | Yield |
|---|---|
| With iodine at 80℃; for 0.5h; Ionic liquid; | 98% |
| With aluminum oxide; 2,3-dicyano-5,6-dichloro-p-benzoquinone for 0.15h; Condensation; oxidation; microwave irradiation; | 83% |
-
-
6287-38-3
3,4-dichlorobenzaldehyde

-
-
146744-39-0, 171426-66-7
1-(3,4-dichlorophenyl)but-3-en-1-ol

| Conditions | Yield |
|---|---|
| With bismuth(III) chloride for 0.0333333h; Irradiation; | 98% |

| Conditions | Yield |
|---|---|
| With sodium carbonate In water at 70℃; for 4h; | 98% |
| With sodium hydroxide In water at 0 - 25℃; for 24h; Inert atmosphere; | 46% |
3,4-Dichlorobenzaldehyde Chemical Properties
3,4-Dichlorobenzaldehyde (6287-38-3) is also named as OTAVA-BB BB7018801961 ; 3,4-DCAD;AKOS BBS-00000836 ; Labotest-BB LT00932384 ; 3,4-dichlorobenzenecarboxaldehyde ; 3,4-Dichlorobenzaldehyde,95% ; Benzaldehyde, 3,4-dichloro- ; CCRIS 6015 ; D56608_ALDRICH ; MLS002415698 ; NSC8763 ,and so on. 3,4-Dichlorobenzaldehyde (6287-38-3) is white to slightly yellow low melting solid.
IUPAC Name: 3,4-Dichlorobenzaldehyde
CAS: 6287-38-3
Molecular Formula: C7H4Cl2O
Molecular Weight: 175.01
Molecular structure: 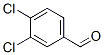
EINECS: 228-520-5
Melting point: 39-42 °C(lit.)
Storage temp.: 0-6°C
Water Solubility: insoluble
Sensitive: Air Sensitive
BRN: 636379
ACD/LogD (pH 5.5): 0.41
ACD/LogD (pH 7.4): 1.44
ACD/BCF (pH 5.5): 1
ACD/BCF (pH 7.4): 1.41
ACD/KOC (pH 5.5): 1
ACD/KOC (pH 7.4): 6.25
H bond acceptors: 4
H bond donors: 1
Freely Rotating Bonds: 9
Index of Refraction: 1.631
Molar Refractivity: 117.36 cm3
Molar Volume: 329 cm3
Polarizability: 46.52 10-24cm3
Surface Tension: 47.6 dyne/cm
Density: 1.172 g/cm3
Flash Point: 287.7 °C
Enthalpy of Vaporization: 83.26 kJ/mol
Boiling Point: 552.1 °C at 760 mmHg
Vapour Pressure: 3.11E-12 mmHg at 25°C
3,4-Dichlorobenzaldehyde Uses
1. 3,4-Dichlorobenzaldehyde (6287-38-3) is commonly used as dye intermediate.
2. 3,4-Dichlorobenzaldehyde (6287-38-3) is organic intermediate to produce medicine.
3,4-Dichlorobenzaldehyde Safety Profile
Hazard Codes: C,Xi ![]()
![]()
Risk Statements: 34-36/37/38 (34: Causes burns 36/37/38: Irritating to eyes, respiratory system and skin)
Safety Statements: 26-27-28-36/37/39-45 (26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice 28: After contact with skin, wash immediately with plenty of ... (to be specified by the manufacturer) 36/37/39: Wear suitable protective clothing, gloves and eye/face protection 45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible))
RIDADR: UN 1759 8/PG 2
WGK Germany: 3
F: 9-23
Hazard Note: Irritant/Air Sensitive
HazardClass: 8
PackingGroup: III
3,4-Dichlorobenzaldehyde Specification
Removal in wastewater treatment of 3,4-Dichlorobenzaldehyde (6287-38-3) can be stated as follows:
Total removal:85.90 percent
Total biodegradation:0.73 percent
Total sludge adsorption:85.17 percent
Total to Air:0.00 percent
(using 10000 hr Bio P,A,S)
Related Products
- 3,10-Diaminotricyclo(5.2.1.0(sup 2,6))decane
- 3,10-Dinitrophenanthrene
- 3-((10-ETHYL-11-(p-HYDROXYPHENYL)DIBENZ-(B,F)OXEPIN-3-YL)OXY)-1,2-PROPANEDIOL HYDRATE (4:1)
- 3-(1,1,2,2-Tetrafluoroethoxy)aniline
- 3-(1,1,2,2-Tetrafluoroethoxy)benzaldehyde
- 3-(1,1,2,2-Tetrafluoroethoxy)bromobenzene
- 3-(1,1,2,2-Tetrafluoroethoxy)toluene
- 3-[1,1'-Biphenyl]-4-yl-1,2,3,4-tetrahydro-1-naphthol
- 3,11-Dichloro-6,11-dihydro-6-methyldibenzo[c,f][1,2]thiazepine 5,5-dioxide
- 3-[(1,1-Dimethyl-2-hydroxyethyl)amino]-2-hydroxypropanesulfonicacid
- 628-73-9
- 6287-40-7
- 62874-44-6
- 62875-07-4
- 628-76-2
- 6287-69-0
- 628-77-3
- 6287-79-2
- 62880-67-5
- 628-81-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View