-
Name
N-METHYL-1,3-PROPANEDIAMINE
- EINECS 228-544-6
- CAS No. 6291-84-5
- Article Data12
- CAS DataBase
- Density 0.823 g/cm3
- Solubility Miscible with water
- Melting Point -72 °C
- Formula C4H12N2
- Boiling Point 140 °C at 760 mmHg
- Molecular Weight 88.1527
- Flash Point 35.6 °C
- Transport Information UN 2734
- Appearance Colourless Liquid with Ammonia odour
- Safety 16-26-36/37-45
- Risk Codes 10-20/21/22-34
-
Molecular Structure
-
Hazard Symbols
 F,
F, C,
C, Xi
Xi
- Synonyms 1,3-Propanediamine,N-methyl- (6CI,7CI,8CI,9CI);(3-Aminopropyl)methylamine;1-(N-Methylamino)-3-aminopropane;1-Amino-3-(methylamino)propane;3-(Methylamino)-1-propylamine;3-(Methylamino)propylamine;3-Amino-1-(methylamino)propane;Koei 3306;Methylaminopropylamine;N-(3-Aminopropyl)-N-methylamine;N-(3-Aminopropyl)methylamine;N-Methyl-1,3-diaminopropane;N-Methyl-1,3-propanediamine;N-Methyl-1,3-propylenediamine;N-Methylpropylylenediamine;N-Methyltrimethylenediamine;N1-Methylpropane-1,3-diamine;NSC 8160;
- PSA 38.05000
- LogP 0.64580
Synthetic route

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether Solvent; Reflux; | 79.1% |
| With lithium aluminium tetrahydride; diethyl ether | |
| With ammonia; nickel at 120℃; under 128714 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In diethyl ether Solvent; Reflux; | 79.1% |
| With lithium aluminium tetrahydride; diethyl ether | |
| With ammonia; nickel at 120℃; under 128714 Torr; Hydrogenation; |

| Conditions | Yield |
|---|---|
| With hydrogen; nickel In methanol |
-
-
72565-83-4
2-ethylsulfanyl-3-methyl-3H-pyrimidin-4-one

-
-
64-17-5
ethanol

-
-
6291-84-5
N-Methyl-1,3-propanediamine


-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With ethanol; sodium |

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With water; sodium hydrogensulfite |

| Conditions | Yield |
|---|---|
| at 120℃; under 125036 Torr; |

-
-
67-56-1
methanol

-
-
109-76-2
Trimethylenediamine

-
A
-
109-55-7
1-amino-3-(dimethylamino)propane

-
B
-
111-33-1
1,3-bis(methylamino)propane

-
C
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With Cs-P-Si at 300℃; under 61504.9 Torr; |


| Conditions | Yield |
|---|---|
| 100% |
-
-
468741-39-1
(+/-)-2-{4-[2-(3-chloro-phenyl)-2-hydroxy-ethylamino]-2-oxo-1,2-dihydro-pyridin-3-yl}-7-methyl-3H-benzimidazole-5-carboximidic ethyl ester

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| In ethanol Heating; | 100% |

| Conditions | Yield |
|---|---|
| at 20℃; | 100% |
-
-
78-84-2
isobutyraldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
70231-94-6
1-methyl-2-(1-methylethyl)-hexahydropyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane at -78 - 23℃; | 99% |
| In toluene |
-
-
123018-64-4
4,4-dipropyl-1-carboxycyclohexane-1-acetic acid anhydride

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
130065-96-2
2-<3-(methylamino)propyl>-8,8-dipropyl-2-azaspiro<4.5>decane-1,3-dione

| Conditions | Yield |
|---|---|
| In toluene Heating; | 99% |
-
-
14470-28-1
mono-4-methoxytrityl chloride

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
1599485-90-1
C24H28N2O

| Conditions | Yield |
|---|---|
| In diethyl ether; dichloromethane for 2h; | 99% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 85℃; | 98.5% |

| Conditions | Yield |
|---|---|
| at 80 - 145℃; for 5.5h; Inert atmosphere; | 98.3% |
| at 80 - 100℃; | 62% |
| In neat (no solvent) at 120℃; for 2h; | 60% |
-
-
492-73-9
2,2'-Pyridil

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
34968-55-3
N-(3-(methylamino)propyl)picolinamide

| Conditions | Yield |
|---|---|
| With Phenazin; 4-ethyl-1-methyl-4H-[1,2,4]-triazol-1-ium iodide; 1,8-diazabicyclo[5.4.0]undec-7-ene In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; Sealed tube; chemoselective reaction; | 98% |
-
-
7655-02-9
2,2-bis(thiophenoxy)-4,4,6,6-tetrachlorocyclotriphosphazatriene

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 97.6% |
-
-
13636-88-9
3,3-bis(methylthio)-1-phenylprop-2-en-1-one

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
124927-52-2
2-(benzoylmethylene)-1-methylhexahydropyrimidine

| Conditions | Yield |
|---|---|
| In ethanol for 12h; Heating; | 97% |
-
-
90-02-8
salicylaldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
101512-38-3
2-((3-methylamino-propylimino)-methyl)-phenol

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 0.5h; | 97% |
| In methanol at 20℃; for 3h; | 82% |
| In methanol for 1h; Reflux; |
-
-
107-20-0
2-chloroethanal

-
-
298-14-6
potassium hydrogencarbonate

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
1580482-35-4
(RS)-1-methylhexahydro[1,3]oxazolo[3,4-a]pyrimidin-6-one

| Conditions | Yield |
|---|---|
| In water at 20℃; for 10h; Green chemistry; | 97% |


-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| Stage #1: 2-(3-cyanobenzyloxy)-4-(2-methyl-(1,1’-diphenyl)-3-yl-methoxyl)benzaldehyde; N-Methyl-1,3-propanediamine With iodine; potassium carbonate In tert-butyl alcohol at 70℃; for 3h; Stage #2: With hydrogenchloride In water; acetonitrile for 0.333333h; | 97% |
-
-
2846-32-4
2,2,4,4-tetrachloro-6,6-diphenylcyclotriphosphazatriene

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
119703-61-6
2,2-(1'-methyl-1',3'-diaminopropane)-4,4-dichloro-6,6-diphenylcyclotriphosphazatriene

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 24h; Inert atmosphere; | 96.6% |
| With triethylamine In dichloromethane | 45% |
-
-
100-52-7
benzaldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
124821-12-1
1-methyl-2-phenylhexahydropyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane at -78 - 23℃; | 96% |
| In toluene | |
| With tert-butyl alcohol at 70℃; for 0.5h; |
-
-
100981-05-3
5-[bis(methylthio)methylene]-2,2-dimethyl-1,3-dioxane-4,6-dione

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
130178-53-9
isopropylidene (1-methyl-2-hexahydropyrimidinylidene)malonate

| Conditions | Yield |
|---|---|
| In ethanol for 3h; Heating; | 96% |
-
-
123-38-6
propionaldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
61327-71-7
2-ethyl-1-methyl-hexahydro-pyrimidine

| Conditions | Yield |
|---|---|
| With potassium carbonate In dichloromethane at -78 - 23℃; | 96% |
-
-
121781-82-6
5-(1-methoxyethylene) Meldrum's acid

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| In acetonitrile for 20h; Heating; | 96% |
-
-
75-05-8
acetonitrile

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
4271-96-9
1,2-dimethyl-1,4,5,6-tetrahydropyrimidine

| Conditions | Yield |
|---|---|
| With thioacetamide at 90 - 110℃; for 6h; Temperature; Reagent/catalyst; Large scale; | 96% |
| Stage #1: acetonitrile; N-Methyl-1,3-propanediamine With zinc(II) chloride for 24h; Inert atmosphere; Reflux; Stage #2: Inert atmosphere; | 45% |
-
-
19652-32-5
3-bromo-5-chlorosalicylaldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| In methanol at 50℃; for 1h; | 96% |
-
-
1101183-58-7
2-isocyanobenzaldehyde

-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With pyridinium p-toluenesulfonate In dimethyl sulfoxide at 25℃; Molecular sieve; | 96% |
-
-
4637-24-5
N,N-dimethyl-formamide dimethyl acetal

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
2304-03-2
1-methyl-1,4,5,6-tetrahydropyrimidine

| Conditions | Yield |
|---|---|
| at 90 - 110℃; for 1h; | 95% |
| In toluene at 80℃; for 24h; | 94% |

| Conditions | Yield |
|---|---|
| In 1-methyl-pyrrolidin-2-one for 120h; | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 24h; | 95% |
-
-
1186467-83-3
(E)-methyl 3-(3,5-dibromo-4-methoxyphenyl)-2-(hydroxyimino)propanoate

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
1338586-56-3
(E)-3-(3,5-dibromo-4-methoxyphenyl)-2-hydroxyimino-N-(3-(methylamino)propyl)propanamide

| Conditions | Yield |
|---|---|
| With methanol at 65℃; Inert atmosphere; Sealed tube; chemoselective reaction; | 95% |

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
1338221-61-6
1,4-bis(6-(1-methyl-1,4,5,6-tetrahydropyrimidin-2-yl)-1H-indol-2-yl)benzene

| Conditions | Yield |
|---|---|
| In ethanol at 20℃; for 120h; | 95% |
-
-
108-10-1
4-methyl-2-pentanone

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
79444-33-0
N-(1,3-dimethylbutylidene)-N'-methyl-propane-1,3-diamine

| Conditions | Yield |
|---|---|
| at 130℃; for 5h; | 95% |
-
-
50-00-0
formaldehyd

-
-
6291-84-5
N-Methyl-1,3-propanediamine

-
-
32743-51-4
1,1-methylenebis(3-methylperhydro-1,3-diazine)

| Conditions | Yield |
|---|---|
| In benzene for 2h; Heating; | 94.3% |
-
-
6291-84-5
N-Methyl-1,3-propanediamine

| Conditions | Yield |
|---|---|
| With nitrogen(II) oxide In acetonitrile under 3800 Torr; for 22h; Ambient temperature; | 94% |
3-Aminopropylmethylamine Specification
The 3-Aminopropylmethylamine, with the CAS registry number 6291-84-5, is also known as N-Methyl-propane-1,3-diamine. It belongs to the product category of Pharmacetical. Its EINECS registry number is 228-544-6. This chemical's molecular formula is C4H12N2 and molecular weight is 88.15. Its IUPAC name and systematic name are the same which is called 3-azaniumylpropyl(methyl)azanium. This chemical is clear liquid.
Physical properties of 3-Aminopropylmethylamine: (1)ACD/LogP: -0.79; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -4.88; (4)ACD/LogD (pH 7.4): -4.68; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 1; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 4; (12)Index of Refraction: 1.43; (13)Molar Refractivity: 27.71 cm3; (14)Molar Volume: 107 cm3; (15)Surface Tension: 28.9 dyne/cm; (16)Density: 0.823 g/cm3; (17)Melting Point: -72 °C; (18)Flash Point: 35.6 °C; (19)Enthalpy of Vaporization: 37.72 kJ/mol; (20)Boiling Point: 140 °C at 760 mmHg; (21)Vapour Pressure: 6.26 mmHg at 25°C.
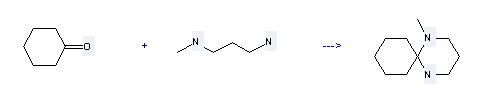
Uses of 3-Aminopropylmethylamine: it can be used to produce 1-methyl-1,5-diaza-spiro[5.5]undecane. This reaction will need reagent toluene-p-sulfonic acid and solvent toluene. The yield is about 85%.
When you are using this chemical, please be cautious about it as the following:
This chemical may catch fire in contact with air. It only need brief contact with an ignition source and it has a very low flash point or evolve highly flammable gases in contact with water. This chemical may destroy living tissue on contact and may cause inflammation to the skin or other mucous membranes. It is harmful by inhalation, in contact with skin and if swallowed. You should keep away from sources of ignition - No smoking. In case of contact with eyes, you should rinse immediately with plenty of water and seek medical advice. Whenever you will contact it, please wear suitable protective clothing and gloves.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: C[NH2+]CCC[NH3+]
(2)InChI: InChI=1S/C4H12N2/c1-6-4-2-3-5/h6H,2-5H2,1H3/p+2
(3)InChIKey: QHJABUZHRJTCAR-UHFFFAOYSA-P
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | oral | 282mg/kg (282mg/kg) | KIDNEY, URETER, AND BLADDER: HEMATURIA | National Technical Information Service. Vol. OTS0555335, |
| rat | LC50 | inhalation | > 189mg/m3/6H (189mg/m3) | National Technical Information Service. Vol. OTS0555335, | |
| rat | LD50 | oral | 951mg/kg (951mg/kg) | KIDNEY, URETER, AND BLADDER: HEMATURIA | National Technical Information Service. Vol. OTS0555335, |
Related Products
- 3-Aminopropylmethylamine
- 6291-85-6
- 6291-89-0
- 629-19-6
- 6291-99-2
- 62922-45-6
- 62922-46-7
- 6292-36-0
- 62924-59-8
- 62924-70-3
- 629-25-4
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View