-
Name
3-Methyl-2-pyrazolin-5-one
- EINECS 203-565-3
- CAS No. 108-26-9
- Article Data114
- CAS DataBase
- Density 1.33 g/cm3
- Solubility Ethanol: slightly soluble
- Melting Point 220-224 ºC
- Formula C4H6 N2 O
- Boiling Point 309.7oC at 760 mmHg
- Molecular Weight 98.1044
- Flash Point 141.1oC
- Transport Information
- Appearance pale white crystal powder
- Safety Moderately toxic by unspecified route. When heated to decomposition it emits toxic vapors of NOx.
- Risk Codes 36/37/38
-
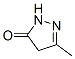
Molecular Structure
- Hazard Symbols
- Synonyms 2-Pyrazolin-5-one,3-methyl- (6CI,7CI,8CI); 2,4-Dihydro-5-methyl-3H-pyrazol-3-one;3-Methyl-1H-4,5-dihydropyrazol-5-one; 3-Methyl-2-pyrazolin-5-one;3-Methyl-5-oxo-2-pyrazoline; 3-Methyl-D2-pyrazol-5-one
- PSA 41.46000
- LogP -0.35340
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrazine In tetrahydrofuran; ethanol at 0 - 60℃; for 26h; Inert atmosphere; | 100% |
| With hydrazine hydrate at 20℃; for 0.0333333h; Knorr Pyrazole Synthesis; | 100% |
| With hydrazine for 0.00833333h; | 94% |

| Conditions | Yield |
|---|---|
| With barium(II) hydroxide In water at 26℃; for 0.75h; Green chemistry; | 100% |

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In methanol at 50℃; for 3h; Temperature; Inert atmosphere; | 97.3% |
| With hydrazine In ethanol |
-
-
27991-08-8
N-acetoacetylphenylhydroxyloamine

-
A
-
4329-75-3
N,N'-bis(isonicotinoyl)hydrazine

-
B
-
100-65-2
N-Phenylhydroxylamine

-
C
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
D
-
495-48-7
azoxybenzene

| Conditions | Yield |
|---|---|
| With isoniazid; triethylamine In chloroform for 24h; Heating; Further byproducts given; | A 39.5% B 32.7% C 90% D 22% |

-
-
27991-08-8
N-acetoacetylphenylhydroxyloamine

-
-
54-85-3
isoniazid

-
A
-
4329-75-3
N,N'-bis(isonicotinoyl)hydrazine

-
B
-
100-65-2
N-Phenylhydroxylamine

-
C
-
143997-57-3
N-hydroxy-N-phenylisonicotinamide

-
D
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| triethylamine In chloroform for 24h; Heating; Further byproducts given; | A 39.5% B 32.7% C 23% D 90% |

-
-
54-85-3
isoniazid

-
A
-
4329-75-3
N,N'-bis(isonicotinoyl)hydrazine

-
B
-
100-65-2
N-Phenylhydroxylamine

-
C
-
143997-57-3
N-hydroxy-N-phenylisonicotinamide

-
D
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With N,O-diacetylphenylhydroxylamine; triethylamine In chloroform for 24h; Heating; Further byproducts given; | A 39.5% B 32.7% C 23% D 90% |


| Conditions | Yield |
|---|---|
| In ethanol Condensation; Cyclization; | 87% |
-
-
116206-94-1
ethyl 2-acetyl-4,4,5,5,6,6,7,7,7-nonafluoro-3-oxoheptanoate

-
-
100-63-0
phenylhydrazine

-
A
-
77146-67-9
2,2,3,3,4,4,5,5,5-Nonafluoro-pentanoic acid N'-phenyl-hydrazide

-
B
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In diethyl ether at -30 - -25℃; for 1h; | A 84% B n/a |

-
-
115479-07-7
2-Acetyl-4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluoro-3-oxo-nonanoic acid ethyl ester

-
-
100-63-0
phenylhydrazine

-
A
-
77146-68-0
2,2,3,3,4,4,5,5,6,6,7,7,7-Tridecafluoro-heptanoic acid N'-phenyl-hydrazide

-
B
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In diethyl ether at -30 - -25℃; for 1h; | A 79% B n/a |

-
-
141-97-9
ethyl acetoacetate

-
-
269727-55-1
N-d-pseudoephedrinylacetic acid hydrazide

-
A
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
B
-
206256-43-1
(5S,6S)-4,5-dimethyl-6-phenyl-2-morpholone

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 10h; Condensation; cyclization; elimination; Heating; | A n/a B 68% |

-
-
100-63-0
phenylhydrazine

-
-
603-69-0
ethyl 2-acetylacetoacetate

-
A
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
B
-
114-83-0
1-acetyl-2-phenylhydrazine

| Conditions | Yield |
|---|---|
| In diethyl ether at -30 - -25℃; | A n/a B 64% |

-
-
141-97-9
ethyl acetoacetate

-
-
269727-54-0
N-l-ephedrinylacetic acid hydrazide

-
A
-
130753-28-5
(5S,6R)-4,5-dimethyl-6-phenylmorpholin-2-one

-
B
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 10h; Condensation; cyclization; elimination; Heating; | A 62% B n/a |


-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With hydrazine In dichloromethane at 20℃; for 19h; | 50% |
-
-
115479-07-7
2-Acetyl-4,4,5,5,6,6,7,7,8,8,9,9,9-tridecafluoro-3-oxo-nonanoic acid ethyl ester

-
-
100-63-0
phenylhydrazine

-
A
-
77146-68-0
2,2,3,3,4,4,5,5,6,6,7,7,7-Tridecafluoro-heptanoic acid N'-phenyl-hydrazide

-
B
-
119403-56-4
5-Methyl-1-phenyl-3-tridecafluorohexyl-1H-pyrazole-4-carboxylic acid ethyl ester

-
C
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In ethanol at -30 - -25℃; for 1h; | A n/a B 27% C n/a |

-
-
896109-93-6
ethyl 2-(1-adamantylcarbonyl)-3-oxobutanoate

-
A
-
496938-13-7
3-(1-adamantyl)-4,5-dihydro-1H-pyrazol-5-one

-
B
-
17846-15-0
adamantane-1-carbohydrazide

-
C
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With hydrazine hydrate In ethanol Cooling; | A 25% B 24% C 11% |

-
-
116206-94-1
ethyl 2-acetyl-4,4,5,5,6,6,7,7,7-nonafluoro-3-oxoheptanoate

-
-
100-63-0
phenylhydrazine

-
A
-
77146-67-9
2,2,3,3,4,4,5,5,5-Nonafluoro-pentanoic acid N'-phenyl-hydrazide

-
B
-
119403-55-3
5-Methyl-3-nonafluorobutyl-1-phenyl-1H-pyrazole-4-carboxylic acid ethyl ester

-
C
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In ethanol at -30 - -25℃; for 1h; | A n/a B 14% C n/a |

-
-
89179-80-6
3-methyl-5-oxo-3-pyrazolin-1-carboxamide

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| geht allmaehlich in 3-Methyl-pyrazolon-(5) ueber; |

| Conditions | Yield |
|---|---|
| at 120℃; |
-
-
3075-23-8
S-ethyl acetothioacetate

-
A
-
4344-87-0
1,2-dihydro-5-methyl-3H-pyrazol-3-one

-
B
-
78024-98-3
5-Hydroxy-5-methyl-pyrazolidin-3-one

-
C
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
D
-
75-08-1
ethanethiol

| Conditions | Yield |
|---|---|
| With hydrazine In methanol; water at 30℃; not separated; | |
| With hydrazine In methanol; water at 30℃; Rate constant; Kinetics; Mechanism; |

-
-
160808-62-8
ethyl acetoacetate (Z)-semicarbazone

-
A
-
110-21-4
hydrazodicarboxamide

-
B
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| In water for 1h; Heating; | A 0.30 g B 0.12 g |
-
-
7664-93-9
sulfuric acid

-
-
3201-21-6
3-ethoxy-5-methyl-1H-pyrazole

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| at 140 - 150℃; |
-
-
89179-80-6
3-methyl-5-oxo-3-pyrazolin-1-carboxamide

-
-
7732-18-5
water

-
-
108-26-9
3-methyl-2-pyrazoline-5-one
-
-
35290-52-9
2-[amino(thioxo)methyl]-5-methyl-2,3-dihydro-1H-3-pyrazolone

-
-
7732-18-5
water

-
-
108-26-9
3-methyl-2-pyrazoline-5-one
-
-
7732-18-5
water

-
-
141-97-9
ethyl acetoacetate

-
-
7803-57-8
hydrazine hydrate

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
35290-52-9
2-[amino(thioxo)methyl]-5-methyl-2,3-dihydro-1H-3-pyrazolone

-
-
108-26-9
3-methyl-2-pyrazoline-5-one
-
-
35290-52-9
2-[amino(thioxo)methyl]-5-methyl-2,3-dihydro-1H-3-pyrazolone

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| at 120℃; im Druckrohr; |

-
-
110-89-4
piperidine

-
-
141-97-9
ethyl acetoacetate

-
-
937-39-3
2-phenylacetylhydrazine

-
A
-
793-25-9
1,2-di(phenylacetyl)hydrazine

-
B
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| at 20℃; |


-
-
7732-18-5
water

-
A
-
7664-41-7
ammonia

-
B
-
141-97-9
ethyl acetoacetate

-
C
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
D
-
108-26-9
3-methyl-2-pyrazoline-5-one


-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| Stage #1: 2-(4,6-dimethyl-3-cyano-2-pyridinylthio)benzenediazonium nitrate; 3-methyl-2-pyrazoline-5-one for 0.0833333h; grinding; Stage #2: With trimethylamine at 20℃; under 375.038 Torr; for 12h; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: α-bromoacetophenone; 3-methyl-2-pyrazoline-5-one at 20℃; for 1h; ball-milling; Stage #2: With sodium carbonate Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| In water at 60℃; for 0.25h; Green chemistry; | 100% |
| With ethanol; sodium acetate at 20℃; for 0.5h; Green chemistry; | 83% |

-
-
3132-99-8
m-bromobenzoic aldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
1032011-01-0
[(3-bromophenyl)(5-hydroxy-3-methyl-1H-pyrazol-4-yl)methyl]malononitrile

| Conditions | Yield |
|---|---|
| With ethanol; sodium acetate at 20℃; for 1h; Green chemistry; | 99% |


| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Solvent; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |
-
-
17630-76-1
5-chloroindole 2,3-dione

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |
-
-
20780-76-1
5-iodoisatin

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With water at 20℃; Green chemistry; | 99% |
-
-
39755-95-8
5-methoxyisatine

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |
-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
169037-23-4
5-trifluoromethoxy-1H-indole-2,3-dione

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 99% |
-
-
104-88-1
4-chlorobenzaldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
89607-39-6
6‐amino‐3‐methyl‐ 4‐(4‐chlorophenyl)‐1,4‐dihydropyrano[2,3‐c]pyrazole‐5‐carbonitrile

| Conditions | Yield |
|---|---|
| With MgFeCrO4 In ethanol; water for 0.0833333h; Catalytic behavior; Solvent; Time; Reflux; | 98% |
| With H3PO4/Al2O3 In neat (no solvent) at 100℃; for 0.1h; Reagent/catalyst; Green chemistry; | 97% |
| With C8H19N2(1+)*HO(1-) In ethanol; water for 0.0833333h; Solvent; Microwave irradiation; | 95% |

-
-
100-52-7
benzaldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
81000-11-5
6-amino-1,4-dihydro-3-methyl-4-phenylpyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 100℃; for 1h; Reagent/catalyst; Temperature; Time; Solvent; Green chemistry; | 98% |
| Stage #1: benzaldehyde; malononitrile In ethanol; water at 20℃; for 0.25h; UV-irradiation; Green chemistry; Stage #2: 3-methyl-2-pyrazoline-5-one In ethanol; water at 20℃; for 0.75h; UV-irradiation; Green chemistry; | 98% |
| With H3PO4/Al2O3 In neat (no solvent) at 100℃; for 0.116667h; Catalytic behavior; Reagent/catalyst; Temperature; Green chemistry; | 97% |

-
-
6363-87-7
1-amino-9,10-dioxo-9,10-dihydro-anthracene-2-carbaldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
107124-90-3
1-amino-2-<(3-methyl-5-oxo-2-pyrazolin-4-ylidene)methyl>anthraquinone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 2h; Heating; | 98% |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid In ethanol for 0.25h; Reagent/catalyst; Temperature; Solvent; Time; Reflux; Green chemistry; | 98% |
| With Santa Barbara Amorphous modified with propylsulfonic acid groups In neat (no solvent) at 120℃; for 0.0666667h; Catalytic behavior; Solvent; Green chemistry; | 95% |
| With sodium dodecyl-sulfate In water for 1h; Heating; | 83.7% |
| In glycerol at 80℃; for 0.05h; Green chemistry; |
-
-
17630-76-1
5-chloroindole 2,3-dione

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
296800-64-1, 713129-95-4
6'‑amino‑3'‑methyl‑2‑oxo‑1'H‑spiro[5‑chloro‑indoline‑3,4'‑pyrano[2,3‑c]pyrazole]‑5'‑carbonitrile

| Conditions | Yield |
|---|---|
| With bovine serum albumin In ethanol; water at 20℃; for 0.25h; Enzymatic reaction; | 98% |
| With N-Sulfonic acid modified poly(styrene-co-maleic anhydride) In ethanol for 0.416667h; Reflux; Green chemistry; regioselective reaction; | 97% |
| With potassium carbonate In water at 20℃; for 0.166667h; | 87.3% |

-
-
18711-13-2
4,7-dichloroisatin

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With water at 20℃; for 0.166667h; Green chemistry; | 98% |

| Conditions | Yield |
|---|---|
| With 1,4-diazabicyclo[2.2.2]octane hydroacetate In water at 80℃; for 0.0833333h; Green chemistry; | 98% |
| With phosphotungstic acid In ethanol for 1h; Reflux; Green chemistry; | 96% |
| With Santa Barbara Amorphous modified with propylsulfonic acid groups In neat (no solvent) at 120℃; Green chemistry; | 87% |
| In glycerol at 80℃; for 0.0333333h; Green chemistry; |
-
-
868-54-2
1,1,3-tricyano-2-amino-1-propene

-
-
90-02-8
salicylaldehyde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

| Conditions | Yield |
|---|---|
| With triethylamine In propan-1-ol at 98℃; for 1h; Solvent; Reagent/catalyst; | 98% |
| With triethylamine In propan-1-ol at 98℃; for 1h; Catalytic behavior; Reagent/catalyst; Solvent; | 98% |

-
-
555-16-8
4-nitrobenzaldehdye

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
89607-34-1
6‐amino‐3‐methyl‐4‐(4‐nitrophenyl)‐1,4‐dihydropyrano[2,3‐c]pyrazole‐5‐carbonitrile

| Conditions | Yield |
|---|---|
| With H3PO4/Al2O3 In neat (no solvent) at 100℃; for 0.0833333h; Reagent/catalyst; Green chemistry; | 97% |
| With piperidine In ethanol at 20℃; for 0.166667h; Green chemistry; | 90% |
| In ethanol; water for 0.333333h; Reflux; Green chemistry; | 88% |

-
-
555-16-8
4-nitrobenzaldehdye

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
89607-34-1, 879451-69-1
6-amino-2,4-dihydro-4-(4-nitrophenyl)-3-methylpyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With propylamine functionalized nanoporous silica In ethanol at 20℃; for 0.25h; Green chemistry; | 97% |
| With 2-carboxy-N,N-diethylethanaminium acetate In neat (no solvent) at 60℃; for 0.1h; | 93% |
| With 1-methyl-3-methylimidazol-3-ium dimethyl phosphate In ethanol; water for 1h; Reflux; | 93% |


| Conditions | Yield |
|---|---|
| With ethanol; sodium acetate at 20℃; for 1h; Time; Green chemistry; | 97% |

-
-
874-42-0
2,4-dichlorobenzaldeyhde

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
297162-27-7, 671771-00-9
6-amino-3-methyl-4-(2,4-dichlorophenyl)-1,4-dihydropyrano[2,3-c]pyrazole-5-carbonitrile

| Conditions | Yield |
|---|---|
| With H3PO4/Al2O3 In neat (no solvent) at 100℃; for 0.0833333h; Reagent/catalyst; Green chemistry; | 97% |
| Stage #1: 2,4-dichlorobenzaldeyhde; malononitrile With triethylamine In ethanol at 80℃; for 0.0333333h; Stage #2: 3-methyl-2-pyrazoline-5-one In ethanol at 80℃; for 0.25h; | 8% |
| With C8H19N2(1+)*HO(1-) In water for 0.0833333h; Microwave irradiation; | |
| In water at 20℃; for 2.5h; Green chemistry; | |
| With aspirin In neat (no solvent) at 80℃; for 0.666667h; Green chemistry; |

-
-
6954-91-2
11H-Indeno[1,2-b]quinoxaline-11-one

-
-
108-26-9
3-methyl-2-pyrazoline-5-one

-
-
109-77-3
malononitrile

-
-
1427302-77-9, 1449513-91-0
3'-amino-7'-methyl-2-oxo-2H,5H'-spiro[(11H)-indeno(1',2'-b)quinoxalin-11,1'-pyrano[2,3-c]pyrazole]-2'-carbonite

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol for 1h; Mechanism; Reflux; | 97% |


| Conditions | Yield |
|---|---|
| With phosphotungstic acid In ethanol for 1h; Reflux; Green chemistry; | 97% |
3-METHYL-2-PYRAZOLIN-5-ONE Chemical Properties
The Molecular Weight of 3-METHYL-2-PYRAZOLIN-5-ONE(108-26-9): 98.1
The Molecular Weight of 3-METHYL-2-PYRAZOLIN-5-ONE(108-26-9):
EINECS: 203-565-3
Melting point: 220-224 °C
Index of Refraction: 1.599
Molar Refractivity: 25.2 cm3
Molar Volume: 73.7 cm3
Polarizability: 9.99 10-24 cm3
Surface Tension: 45.6 dyne/cm
Density: 1.33 g/cm3
Appearance: Yellowish powder
Synonyms: TIMTEC-BB SBB004372;3-Methyl-pyrazolon-(5);52-Pyrazolin-5-one, 3-methyl-;3-METHYL-4,5-DIHYDRO-1H-PYRAZOL-5-ONE;3-METHYL-5-PYRAZOLONE;3-methyl-2-pyrazolin-5-on;-Methyl-2,4-dihydro-3H-pyrazol-3-one;AURORA KA-6219;
3-METHYL-2-PYRAZOLIN-5-ONE Uses
3-METHYL-2-PYRAZOLIN-5-ONE Toxicity Data With Reference
| 1. | unr-rat LDLo:600 mg/kg | BCPCA6 Biochemical Pharmacology. 14 (1965),1325. |
3-METHYL-2-PYRAZOLIN-5-ONE Consensus Reports
3-METHYL-2-PYRAZOLIN-5-ONE Safety Profile
The Risk Statements information of 3-METHYL-2-PYRAZOLIN-5-ONE(108-26-9):
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements information of 3-METHYL-2-PYRAZOLIN-5-ONE(108-26-9):
24/25: Avoid contact with skin and eyes
RTECS: UQ9451500
Related Products
- 3-METHYL-2-PYRAZOLIN-5-ONE
- 108-27-0
- 1082743-32-5
- 108274-33-5
- 1082743-70-1
- 108274-39-1
- 1082766-40-2
- 108-28-1
- 108281-78-3
- 108281-79-4
- 108282-38-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View