-
Name
4'-ETHOXY-2'-HYDROXYACETOPHENONE
- EINECS
- CAS No. 37470-42-1
- Article Data22
- CAS DataBase
- Density 1.127 g/cm3
- Solubility
- Melting Point 40-45 °C(lit.)
- Formula C10H12O3
- Boiling Point 314.7 °C at 760 mmHg
- Molecular Weight 180.203
- Flash Point 124 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Acetophenone, 4'-ethoxy-2'-hydroxy- (6CI);1-(4-Ethoxy-2-hydroxyphenyl)ethanone;2-Acetyl-5-ethoxyphenol;4-Ethoxy-2-hydroxyacetophenone;
- PSA 46.53000
- LogP 1.99350
Synthetic route
-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
75-03-6
ethyl iodide

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone at 80℃; for 6h; | 97% |
| With potassium carbonate In N,N-dimethyl-formamide for 4h; | 63% |
-
-
64-67-5
diethyl sulfate

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 2h; Reflux; | 78% |
| In acetone at 20℃; for 25h; Reflux; |
-
-
74-96-4
ethyl bromide

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 13h; Reflux; | 73.5% |
| With potassium carbonate In acetone for 24h; Heating; Yield given; |
-
-
74-96-4
ethyl bromide

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
829-20-9
2',4'-dimethoxyacetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate |

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With water; silver nitrate und Erwaermen des erhaltenen Silber-Salzes mit Aethyljodid in Aceton; |
-
-
110690-86-3
7-ethoxy-2-methyl-chromen-4-one

-
-
141-52-6
sodium ethanolate

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
141-52-6
sodium ethanolate

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
802294-64-0
propionic acid

-
-
93321-62-1
7-ethoxy-2-phenyl-4H-chromen-4-one

-
-
141-52-6
sodium ethanolate

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
65-85-0
benzoic acid


-
-
141-52-6
sodium ethanolate

-
A
-
103-82-2
phenylacetic acid

-
B
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone


-
-
141-52-6
sodium ethanolate

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
100-09-4
4-methoxybenzoic acid


-
-
141-52-6
sodium ethanolate

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
10435-55-9
4-ethoxy-2-hydroxybenzoic acid

-
C
-
134-11-2
2-ethoxybenzoic acid


-
-
141-52-6
sodium ethanolate

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
621-51-2
3-ethoxybenzoic acid

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
75-03-6
ethyl iodide

-
A
-
22924-18-1
2′,4′-diethoxyacetophenone

-
B
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium carbonate |

-
-
89-84-9
2',4'-dihydroxy-4-acetophenone

-
-
75-03-6
ethyl iodide

-
A
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
B
-
829-20-9
2',4'-dimethoxyacetophenone

| Conditions | Yield |
|---|---|
| With potassium carbonate |

-
-
93321-62-1
7-ethoxy-2-phenyl-4H-chromen-4-one

-
A
-
621-34-1
O-ethyl resorcinol

-
B
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
C
-
65-85-0
benzoic acid

| Conditions | Yield |
|---|---|
| Kalischmelze; |

-
-
108-46-3
recorcinol

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: zinc(II) chloride; hydrogenchloride / diethyl ether / 0.5 h / 0 °C 2: water / 2 h / Reflux 3: potassium carbonate / acetone / 2 h / Reflux View Scheme |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With hydrogen; palladium 10% on activated carbon In methanol for 24h; | 97% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
52191-14-7
2-(2-acetylphenoxy)-1-bromoethane

-
-
635538-05-5
1-(2-acetylphenoxy)-2-(2-acetyl-5-ethoxyphenoxy)ethane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 88% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With pyrrolidine In ethanol at 80℃; for 10h; Kabe Chromanone Synthesis; | 85% |
-
-
110-91-8
morpholine

-
-
50-00-0
formaldehyd

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
A
-
1064287-95-1
1-[4-ethoxy-2-hydroxy-5-(morpholinomethyl)phenyl]ethanone

-
B
-
1064287-96-2
1-[4-ethoxy-2-hydroxy-3-(morpholinomethyl)phenyl]ethanone

| Conditions | Yield |
|---|---|
| In ethanol; water at 120℃; Mannich reaction; | A 83% B 6% |

-
-
1757-42-2, 6195-92-2
3-methyl-cyclopentanone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
1069133-20-5
(1'S,3'R)-7-ethoxy-3'-methylspiro[chroman-2,1'-cyclopentan]-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-cyclopentanone; 1-(4-ethoxy-2-hydroxyphenyl)ethanone With cis-Hyp(tBu)-Thr(tBu)-TentaGel In methanol at 20℃; for 1h; Stage #2: In methanol at 110℃; for 0.183333h; Microwave irradiation; optical yield given as %ee; enantioselective reaction; | 83% |
-
-
3002-81-1
5, 6-dimethyl-1,10-phenanthroline

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 55℃; for 0.2h; pH=7 - 8; | 82% |

-
-
51490-01-8
1-(3,4,5-trimethoxyphenyl)-2-bromoethanone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
1193107-64-0
(6-ethoxy-3-methylbenzofuran-2-yl)(3,4,5-trimethoxyphenyl)methanone

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 18h; Reflux; | 81% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
16420-13-6
N,N-Dimethylthiocarbamoyl chloride

-
-
1239890-34-6
O-2-acetyl-5-ethoxyphenyl N,N-dimethylcarbamothioate

| Conditions | Yield |
|---|---|
| With 1,4-diaza-bicyclo[2.2.2]octane In N,N-dimethyl-formamide at 50℃; for 5h; Inert atmosphere; | 81% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
583-60-8
2-Methylcyclohexanone

-
-
1069133-30-7
C17H22O3

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-ethoxy-2-hydroxyphenyl)ethanone; 2-Methylcyclohexanone With cis-Hyp(tBu)-Thr(tBu)-TentaGel In methanol at 20℃; for 1h; Stage #2: In methanol at 110℃; for 0.183333h; Microwave irradiation; optical yield given as %ee; enantioselective reaction; | 80% |
-
-
366-18-7
[2,2]bipyridinyl

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 55℃; for 0.2h; pH=7 - 8; | 79% |

-
-
1757-42-2, 6195-92-2
3-methyl-cyclopentanone

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
1069133-19-2
C16H20O3

| Conditions | Yield |
|---|---|
| Stage #1: 3-methyl-cyclopentanone; 1-(4-ethoxy-2-hydroxyphenyl)ethanone With trans-Hyp(tBu)-Thr(tBu)-TentaGel In methanol at 20℃; for 1h; Stage #2: In methanol at 110℃; for 0.183333h; Microwave irradiation; optical yield given as %ee; enantioselective reaction; | 78% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
100-52-7
benzaldehyde

-
-
93321-62-1
7-ethoxy-2-phenyl-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| With pyrrolidine; iodine In dimethyl sulfoxide at 150℃; for 12h; | 78% |
-
-
7570-45-8
N-ethyl-3-carbazolealdehyde

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With potassium hydroxide for 0.133333h; Claisen-Schmidt Condensation; Microwave irradiation; | 78% |
| With potassium hydroxide Claisen-Schmidt Condensation; Sealed tube; Microwave irradiation; Green chemistry; | 78% |
| Claisen-Schmidt Condensation; |
-
-
66-71-7
1,10-Phenanthroline

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 55℃; for 0.2h; pH=7 - 8; | 78% |

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
583-60-8
2-Methylcyclohexanone

-
-
1069133-29-4
(1'R,2'S)-7-ethoxy-2'-methylspiro[chroman-2,1'-cyclohexan]-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-ethoxy-2-hydroxyphenyl)ethanone; 2-Methylcyclohexanone With trans-Hyp(tBu)-Thr(tBu)-TentaGel In methanol at 20℃; for 1h; Stage #2: In methanol at 110℃; for 0.183333h; Microwave irradiation; optical yield given as %ee; enantioselective reaction; | 76% |

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
1662-01-7
bathophenanthroline

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 55℃; for 0.2h; pH=7 - 8; | 72% |

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
21382-82-1
(carbethoxyethylidene)triphenylphosphorane

-
-
111244-98-5
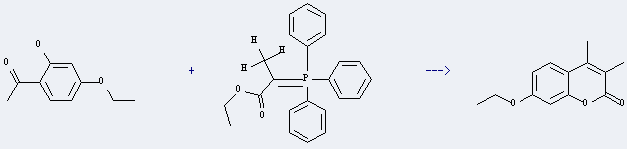
7-ethoxy-3,4-dimethyl-coumarin

| Conditions | Yield |
|---|---|
| at 180℃; for 10h; | 66% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium azide; iodine; sodium hydrogencarbonate In N,N-dimethyl-formamide at 100℃; for 2h; | 66% |

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
7732-18-5
water

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol at 50 - 55℃; for 0.2h; pH=7 - 8; | 65% |

-
-
98-03-3
thiophene-2-carbaldehyde

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
1364733-90-3
7-ethoxy-3-hydroxy-2-(thiophen-2-yl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| Stage #1: thiophene-2-carbaldehyde; 1-(4-ethoxy-2-hydroxyphenyl)ethanone With methanol; sodium hydroxide for 3h; Reflux; Stage #2: With dihydrogen peroxide at 20℃; | 55% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium azide; iodine; sodium hydrogencarbonate In water at 100℃; for 2h; | 54% |
-
-
5381-20-4
benzothiophene-3-carboxaldehyde

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol; water at 20℃; Claisen-Schmidt Condensation; | 52% |
-
-
110-91-8
morpholine

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

| Conditions | Yield |
|---|---|
| With sulfur at 130℃; for 16h; | 52% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
100-52-7
benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-ethoxy-2-hydroxyphenyl)ethanone; benzaldehyde With methanol; sodium hydroxide for 3h; Reflux; Stage #2: With dihydrogen peroxide at 20℃; | 50% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
87199-17-5
4-formylphenylboronic acid,

-
-
1364733-22-1
7-ethoxy-3-hydroxy-2-(4-hydroxyphenyl)-4H-chromen-4-one

| Conditions | Yield |
|---|---|
| Stage #1: 1-(4-ethoxy-2-hydroxyphenyl)ethanone; 4-formylphenylboronic acid, With methanol; sodium hydroxide for 3h; Reflux; Stage #2: With dihydrogen peroxide at 20℃; | 40% |
-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
100-52-7
benzaldehyde

-
-
70668-35-8
4'-ethoxy-2'-hydroxy-trans-chalcone

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol; water at 20℃; for 24h; Aldol Condensation; | 20% |
-
-
75-15-0
carbon disulfide

-
-
37470-42-1
1-(4-ethoxy-2-hydroxyphenyl)ethanone

-
-
173210-12-3
7-Ethoxy-4-hydroxy-2H-1-benzopyran-2-thione

| Conditions | Yield |
|---|---|
| With potassium tert-butylate In benzene Ambient temperature; | 18% |
4'-Ethoxy-2'-hydroxyacetophenone Specification
The Ethanone, 1-(4-ethoxy-2-hydroxyphenyl)- is an organic compound with the formula C10H12O3. The systematic name of this chemical is 1-(4-ethoxy-2-hydroxyphenyl)ethanone. With the CAS registry number 37470-42-1, it is also named as 4'-Ethoxy-2'-hydroxyacetophenone. The product's categories are Aromatic Acetophenones & Derivatives (substituted); C10; Carbonyl Compounds; Ketones.
Physical properties about Ethanone, 1-(4-ethoxy-2-hydroxyphenyl)- are: (1)ACD/LogP: 2.69; (2)ACD/LogD (pH 5.5): 2.69; (3)ACD/LogD (pH 7.4): 2.69; (4)ACD/BCF (pH 5.5): 65.08; (5)ACD/BCF (pH 7.4): 64.84; (6)ACD/KOC (pH 5.5): 691.29; (7)ACD/KOC (pH 7.4): 688.74; (8)#H bond acceptors: 3; (9)#H bond donors: 1; (10)#Freely Rotating Bonds: 4; (11)Polar Surface Area: 35.53 Å2; (12)Index of Refraction: 1.531; (13)Molar Refractivity: 49.47 cm3; (14)Molar Volume: 159.9 cm3; (15)Polarizability: 19.61×10-24cm3; (16)Surface Tension: 40.7 dyne/cm; (17)Density: 1.126 g/cm3; (18)Flash Point: 124 °C; (19)Enthalpy of Vaporization: 57.81 kJ/mol; (20)Boiling Point: 314.7 °C at 760 mmHg; (21)Vapour Pressure: 0.000247 mmHg at 25°C.
Uses of Ethanone, 1-(4-ethoxy-2-hydroxyphenyl)-: it can be used to produce 7-ethoxy-3,4-dimethyl-coumarin at temperature of 180 °C. The reaction time is 10 hours. The yield is about 66%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable protective clothing. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(c1ccc(OCC)cc1O)C
(2)InChI: InChI=1/C10H12O3/c1-3-13-8-4-5-9(7(2)11)10(12)6-8/h4-6,12H,3H2,1-2H3
(3)InChIKey: VBLALYJSGGGWHU-UHFFFAOYAV
(4)Std. InChI: InChI=1S/C10H12O3/c1-3-13-8-4-5-9(7(2)11)10(12)6-8/h4-6,12H,3H2,1-2H3
(5)Std. InChIKey: VBLALYJSGGGWHU-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View