-
Name
4,6-DIMETHYLPYRIMIDINE
- EINECS 216-314-8
- CAS No. 1558-17-4
- Article Data16
- CAS DataBase
- Density 0.98 g/cm3
- Solubility Soluble in water at 20°C.
- Melting Point 25°C
- Formula C6H8N2
- Boiling Point 154 °C at 760 mmHg
- Molecular Weight 108.143
- Flash Point 56 °C
- Transport Information UN 1993 3/PG 3
- Appearance clear yellowish liquid after melting
- Safety 23-24/25
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms NSC 60686;
- PSA 25.78000
- LogP 1.09340
Synthetic route
-
-
123-32-0
2,5-dimethyl-pyrazine

-
A
-
22868-76-4
2,5-dimethylpyrimidine

-
B
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| under 1 - 1.5 Torr; for 0.0833333h; Irradiation; | A 72% B 26% |
-
-
82619-54-3
N-Benzyl-4,6-dimethylpyrimidinium bromide

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With ammonia at -33℃; for 2h; Product distribution; Mechanism; | 65% |
-
-
82619-55-4
N-(p-nitrophenyl)-4,6-dimethylpyrimidinium bromide

-
A
-
1558-17-4
4,6-dimethylpyrimidine

-
B
-
7409-30-5
p-nitrobenzylamine

| Conditions | Yield |
|---|---|
| With ammonia at -33℃; for 2h; Product distribution; Mechanism; | A 65% B 37% |
-
-
22868-76-4
2,5-dimethylpyrimidine

-
A
-
123-32-0
2,5-dimethyl-pyrazine

-
B
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| under 1 - 1.5 Torr; for 0.0833333h; Irradiation; | A 62% B 31% |

| Conditions | Yield |
|---|---|
| With water; zinc | |
| With palladium on activated charcoal; sodium acetate; acetic acid Hydrogenation; | |
| With palladium on activated charcoal; ethanol; sodium acetate under 5148.6 Torr; Hydrogenation; | |
| Multi-step reaction with 2 steps 1: N2H4 * H2O 2: aqueous CuSO4 View Scheme |
-
-
67-56-1
methanol

-
-
22126-16-5
4,6-dimethyl-2-pyrimidinecarbonitrile

-
A
-
1558-17-4
4,6-dimethylpyrimidine

-
B
-
27427-89-0
methyl 4,6-dimethylpyrimidine-2-carboxylate

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water |

-
-
60420-76-0
4,6-dimethyl-2-pyrimidinecarboxylic acid

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| Destillieren; |
-
-
23906-13-0
2-hydrazino-4,6-dimethylpyrimidine

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With copper(II) sulfate |
-
-
62501-45-5
4,6-dimethylpyrimidine-2(1H)-thione hydrochloride

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; nickel |
-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
123-54-6
acetylacetone

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With water at 180 - 190℃; | |
| at 220 - 240℃; |
-
-
289-95-2
PYRIMIDINE

-
-
75-91-2
tert.-butylhydroperoxide

-
A
-
3438-46-8
4-Methylpyrimidine

-
B
-
5053-43-0
2-methylpyrimidine

-
C
-
14331-54-5
2,4-dimethylpyrimidine

-
D
-
1558-17-4
4,6-dimethylpyrimidine

-
E
-
22114-27-8
2,4,6-trimethylpyrimidine

| Conditions | Yield |
|---|---|
| With sulfuric acid; iron(II) sulfate In water at 20 - 25℃; for 0.5h; Product distribution; various radical source ratios, varipus conversion and produts yield; |

-
-
289-95-2
PYRIMIDINE

-
-
64-19-7
acetic acid

-
A
-
3438-46-8
4-Methylpyrimidine

-
B
-
5053-43-0
2-methylpyrimidine

-
C
-
14331-54-5
2,4-dimethylpyrimidine

-
D
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With ammonium thiosulphate; silver nitrate at 90℃; for 2h; Product distribution; various radical source ratios, varipus conversion and produts yield; |

-
-
289-95-2
PYRIMIDINE

-
-
67-68-5
dimethyl sulfoxide

-
A
-
3438-46-8
4-Methylpyrimidine

-
B
-
5053-43-0
2-methylpyrimidine

-
C
-
14331-54-5
2,4-dimethylpyrimidine

-
D
-
1558-17-4
4,6-dimethylpyrimidine

-
E
-
22114-27-8
2,4,6-trimethylpyrimidine

| Conditions | Yield |
|---|---|
| With sulfuric acid; dihydrogen peroxide; iron(II) sulfate In water Product distribution; Ambient temperature; various radical source ratios, varipus conversion and produts yield; |

-
-
3438-46-8
4-Methylpyrimidine

-
-
75-91-2
tert.-butylhydroperoxide

-
A
-
14331-54-5
2,4-dimethylpyrimidine

-
B
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| With sulfuric acid; iron(II) sulfate In water at 20 - 25℃; for 0.5h; | A 32 % Turnov. B 65 % Turnov. |

-
-
5053-43-0
2-methylpyrimidine

-
-
75-91-2
tert.-butylhydroperoxide

-
A
-
14331-54-5
2,4-dimethylpyrimidine

-
B
-
1558-17-4
4,6-dimethylpyrimidine

-
C
-
22114-27-8
2,4,6-trimethylpyrimidine

| Conditions | Yield |
|---|---|
| With sulfuric acid; iron(II) sulfate In water at 20 - 25℃; for 0.5h; | A 32 % Turnov. B 65 % Turnov. C 3 % Turnov. |


| Conditions | Yield |
|---|---|
| In dimethylsulfoxide-d6 at 22 - 28℃; Mechanism; determined of intermedier by 13C nmr; |

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| bei der Destillation; |

-
-
77767-94-3
(4,6-dimethylpyrimid-2′-yl)-trimethylammonium chloride

-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: acetamide 2: HCl; water View Scheme |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
459-46-1
4-Fluorobenzyl bromide

-
-
329983-94-0
4-[2-(4-fluorophenyl)ethyl]-6-methylpyrimidine

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With n-butyllithium In tetrahydrofuran; hexane at -78℃; Stage #2: 4-Fluorobenzyl bromide In tetrahydrofuran; hexane at -78℃; Further stages.; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With n-butyllithium In tetrahydrofuran; hexane at -78℃; Stage #2: benzyl bromide In tetrahydrofuran; hexane at -78℃; Further stages.; | 99% |
| With n-butyllithium; ammonium chloride In tetrahydrofuran |

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With n-butyllithium In tetrahydrofuran at -78℃; for 0.333333h; Stage #2: carbon dioxide In tetrahydrofuran at -78 - 15℃; for 1h; | 97% |
| Stage #1: 4,6-dimethylpyrimidine With n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.333333h; Stage #2: carbon dioxide In tetrahydrofuran; hexane at 15℃; for 1h; | 97% |
| Stage #1: 4,6-dimethylpyrimidine With [2,2]bipyridinyl; n-butyllithium In tetrahydrofuran; hexane at -78℃; for 0.333333h; Inert atmosphere; Stage #2: carbon dioxide In tetrahydrofuran; hexane at -78 - 20℃; for 1.25h; |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
1192-58-1
N-methylpyrrole aldehyde

-
-
1154426-21-7
(E,E)-4,6-bis[2-(1-methyl-1H-pyrrol-2-yl)vinyl]pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 95% |
| With Aliquat 336; sodium hydroxide at 20 - 110℃; for 4h; | 40% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
1233385-56-2
Au(chloride)3(4,6-dimethylpyrimidine)

| Conditions | Yield |
|---|---|
| In methanol; water dropwise addn. of soln. of N compd. in methanol to aq. soln. of Au compd.; filtration, washing with water, drying under vacuum; | 95% |

| Conditions | Yield |
|---|---|
| With ammonium peroxydisulfate; sulfuric acid In water for 5h; Heating; | 93% |
-
-
98-03-3
thiophene-2-carbaldehyde

-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
1154426-26-2
(E,E)-4,6-bis[2-(thiophen-2-yl)vinyl]pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 93% |
-
-
498-62-4
3-thiophene carboxaldehyde

-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
1154426-36-4
(E,E)-4,6-bis[2-(thiophen-3-yl)vinyl]pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 93% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
10338-57-5
4-(piperidin-1-yl)benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With potassium tert-butylate In N,N-dimethyl-formamide at 80℃; for 0.25h; Stage #2: 4-(piperidin-1-yl)benzaldehyde In N,N-dimethyl-formamide at 80℃; for 4h; | 92% |

| Conditions | Yield |
|---|---|
| With sodium persulfate; [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate; trifluoroacetic acid In water; acetonitrile at 23℃; for 8h; Minisci Aromatic Substitution; Inert atmosphere; Irradiation; regioselective reaction; | 91% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
100-52-7
benzaldehyde

-
-
68763-05-3, 36272-52-3
(E,E)-4,6-bis-styrylpyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate for 1h; Heating; | 90% |
| With sodium hydroxide In water for 1h; Reflux; | 79% |
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water Reflux; | 76% |
| With sodium hydroxide; Aliquat 336 for 1h; Heating; |

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate for 1h; Heating; | 90% |
| With sodium hydroxide In water for 2h; Inert atmosphere; Reflux; | 90% |
| With sodium hydroxide; Aliquat 336 for 1h; Heating; |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
274249-48-8
trisilver(I) tris(3,5-bis(trifluoromethyl)pyrazolate)

| Conditions | Yield |
|---|---|
| In toluene at 20℃; for 12h; Inert atmosphere; Schlenk technique; | 90% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
100-10-7
4-dimethylamino-benzaldehyde

-
-
1154426-01-3
4,4′-(1E,1′E)-2,2′-(pyrimidine-4,6-diyl)bis(ethene-2,1-diyl)bis(N,N-dimethylaniline)

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With potassium tert-butylate In N,N-dimethyl-formamide at 80℃; for 0.25h; Stage #2: 4-dimethylamino-benzaldehyde In N,N-dimethyl-formamide at 80℃; for 4h; | 89% |
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 77% |
| With sodium hydroxide In water for 2h; Inert atmosphere; Reflux; | 77% |
| With Aliquat 336; sodium hydroxide at 20 - 110℃; for 4h; | 20% |

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With potassium tert-butylate In N,N-dimethyl-formamide at 80℃; for 0.25h; Stage #2: 4-(4-morpholinyl)benzaldehyde In N,N-dimethyl-formamide at 80℃; for 4h; | 88% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
4707-71-5
Phenanthrene-9-carboxaldehyde

-
-
1154425-59-8
(E,E)-4,6-bis[2-(phenanthren-9-yl)vinyl]pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 86% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
51980-54-2
4-(pyrrolidin-1-yl)benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With potassium tert-butylate In N,N-dimethyl-formamide at 80℃; for 0.25h; Stage #2: 4-(pyrrolidin-1-yl)benzaldehyde In N,N-dimethyl-formamide at 80℃; for 4h; | 86% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
81172-89-6
terephthalaldehyde mono(diethylacetal)

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 for 1h; Heating; | 85% |
| Stage #1: 4,6-dimethylpyrimidine; terephthalaldehyde mono(diethylacetal) With sodium hydroxide In water for 2h; Heating; Stage #2: With hydrogenchloride In acetone at 20℃; for 0.0833333h; | 71% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
77182-73-1
4-[(4-methoxyphenyl)methoxy]benzaldehyde

-
-
1396036-52-4
(E,E)-4,6-bis[4'-(4''-methoxybenzyloxy)styryl]pyrimidine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water Reflux; | 84% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
27913-99-1
4-(4-methylpiperazin-1-yl) benzaldehyde

| Conditions | Yield |
|---|---|
| Stage #1: 4,6-dimethylpyrimidine With potassium tert-butylate In N,N-dimethyl-formamide at 80℃; for 0.25h; Stage #2: 4-(4-methylpiperazin-1-yl) benzaldehyde In N,N-dimethyl-formamide at 80℃; for 4h; | 84% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
15666-84-9
2-phenylperimidine

| Conditions | Yield |
|---|---|
| With polyphosphoric acid at 250℃; | 82% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
5736-94-7
4-(hexyloxy)benzaldehyde

-
-
1154425-13-4
(E,E)-4,6-bis(4-hexyloxystyryl)pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 81% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
72785-29-6
4-methoxy-3-((4-methoxybenzyl)oxy)benzaldehyde

-
-
1236355-55-7
(E,E)-4,6-bis[4'-methoxy-3'-(4''-methoxybenzyloxy)styryl]pyrimidine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water Reflux; | 81% |
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate In water Reflux; |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
129047-38-7
3-methoxy 4-((4-methoxybenzyl)oxy)benzaldehyde

-
-
1396036-62-6
(E,E)-4,6-bis[3'-methoxy-4'-(4''-methoxybenzyloxy)styryl]pyrimidine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water Reflux; | 81% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
81172-89-6
terephthalaldehyde mono(diethylacetal)

| Conditions | Yield |
|---|---|
| With sodium hydroxide; tetra(n-butyl)ammonium hydrogensulfate for 1h; Heating; | 80% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
4701-17-1
5-bromo-2-thiophencarboxaldehyde

-
-
1154426-31-9
(E,E)-4,6-bis[2-(5-bromothiophen-2-yl)vinyl]pyrimidine

| Conditions | Yield |
|---|---|
| With sodium hydroxide; Aliquat 336 In water for 1h; Reflux; | 78% |

| Conditions | Yield |
|---|---|
| With polyphosphoric acid at 250℃; | 78% |
-
-
1558-17-4
4,6-dimethylpyrimidine

| Conditions | Yield |
|---|---|
| In acetone byproducts: AgCl; acetone soln. of AgClO4 added to suspn. of Rh complex in acetone, stirred for 30 min, filtered off AgCl, added amine ligand to filtrate, stirred for 20 min; concd., added hexane, filtered off, washed with hexane, air-dried, elem. anal.; | 76% |

-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
12257-42-0
[(norbornadiene)rhodium(I)chloride]2

-
-
90423-47-5
{(RhCl(2,5-norbornadiene))2(4,6-dimethylpyrimidine)}

| Conditions | Yield |
|---|---|
| In acetone addn. of amine ligand to suspn. of Rh complex in acetone, soln. stirred for 20 min; concd. in vac., added hexane, filtered off, washed with hexane, air-dried, elem. anal.; | 76% |
-
-
1558-17-4
4,6-dimethylpyrimidine

-
-
108781-14-2
3-(4-Methoxybenzyloxy)benzaldehyde

-
-
1396036-57-9
(E,E)-4,6-bis[3'-(4''-methoxybenzyloxy)styryl]pyrimidine

| Conditions | Yield |
|---|---|
| With tetra(n-butyl)ammonium hydrogensulfate; sodium hydroxide In water Reflux; | 76% |
4,6-Dimethylpyrimidine Specification
The Pyrimidine,4,6-dimethyl-, with the CAS registry number 1558-17-4, is also known as NSC60686. It belongs to the product categories of Pyrimidine; Pyridines, Pyrimidines, Purines and Pteredines; Building Blocks; Heterocyclic Building Blocks; Pyrimidines. Its EINECS registry number is 216-314-8. This chemical's molecular formula is C6H8N2 and molecular weight is 108.14. Its IUPAC name is called 4,6-dimethylpyrimidine.
Physical properties of Pyrimidine,4,6-dimethyl-: (1)ACD/LogP: 0.59; (2)ACD/LogD (pH 5.5): 0.59; (3)ACD/LogD (pH 7.4): 0.59; (4)ACD/BCF (pH 5.5): 1.64; (5)ACD/BCF (pH 7.4): 1.64; (6)ACD/KOC (pH 5.5): 49.51; (7)ACD/KOC (pH 7.4): 49.68; (8)#H bond acceptors: 2; (9)Index of Refraction: 1.503; (10)Molar Refractivity: 32.08 cm3; (11)Molar Volume: 108.4 cm3; (12)Surface Tension: 38.9 dyne/cm; (13)Density: 0.997 g/cm3; (14)Flash Point: 56 °C; (15)Enthalpy of Vaporization: 37.47 kJ/mol; (16)Boiling Point: 154 °C at 760 mmHg; (17)Vapour Pressure: 4.17 mmHg at 25°C.
Preparation of Pyrimidine,4,6-dimethyl-: this chemical can be prepared by N-Benzyl-4,6-dimethylpyrimidinium bromide. This reaction will need reagent NH3(l). The reaction time is 2 hours with reaction temperature of -33 °C. The yield is about 65%.
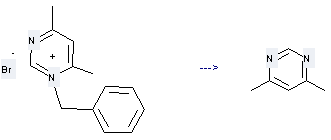
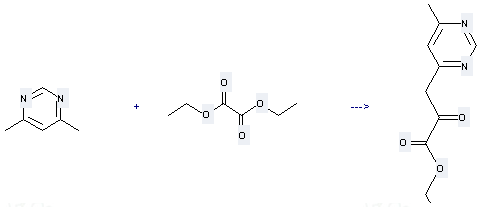
Uses of Pyrimidine,4,6-dimethyl-: it can be used to produce (6-methyl-pyrimidin-4-yl)-pyruvic acid ethyl ester. This reaction will need reagents potassium ethylate and diethyl ether.
When you are using this chemical, please be cautious about it. It is flammable. You should not breathe gas/fumes/vapour/spray (appropriate wording to be specified by the manufacturer). What's more, you must avoid contact with skin and eyes.
You can still convert the following datas into molecular structure:
(1)Canonical SMILES: CC1=CC(=NC=N1)C
(2)InChI: InChI=1S/C6H8N2/c1-5-3-6(2)8-4-7-5/h3-4H,1-2H3
(3)InChIKey: LSBIUXKNVUBKRI-UHFFFAOYSA-N
Related Products
- 4-06-00-02342 (Beilstein Handbook Reference)
- 4,10-Ace-1,2-benzanthracene
- 4,10-Dioxatricyclo[5.2.1.0(2,6)]decan-8-en-3-one
- 4-(1,1,2,2-Tetrafluoroethoxy)benzoicacid
- 4-(1,1,2,2-Tetrafluoroethoxy)chlorobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)nitrobenzene
- 4-(1,1,2,2-Tetrafluoroethoxy)toluene
- 4-(1,1-Difluoropropan-2-yl)benzene-1-sulfonyl chloride
- 4-(1,1-Dioxothiazolidin-2-yl)benzoate
- 4′-(1,2,3,4-TETRAHYDRO-4-(4-HYDROXY-2-OXO-2H-1-BENZOPYRAN-3-YL)-2-NAPHTHALENYL)(1,1′-BIPHENYL)-4-CARBONITRILE, cis-
- 155819-07-1
- 155824-29-6
- 1558-24-3
- 1558-25-4
- 155830-69-6
- 15583-16-1
- 15583-17-2
- 1558-33-4
- 155835-09-9
- 155836-47-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View