-
Name
4-Aminobenzamidine dihydrochloride
- EINECS 219-692-2
- CAS No. 2498-50-2
- Article Data9
- CAS DataBase
- Density 1.26g/cm3
- Solubility
- Melting Point >300 °C(lit.)
- Formula C7H11Cl2N3
- Boiling Point 289.8 °C at 760 mmHg
- Molecular Weight 208.09
- Flash Point 129 °C
- Transport Information
- Appearance white to yellowish fine crystalline powder
- Safety 26-37/39-36
- Risk Codes 36/37/38-22
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  Xn
Xn
- Synonyms Benzamidine,p-amino-, dihydrochloride (6CI,7CI,8CI);Benzenecarboximidamide, 4-amino-,dihydrochloride (9CI);4-Amidinoaniline dihydrochloride;p-Aminobenzamidine dihydrochloride;
- PSA 75.89000
- LogP 3.53810
Synthetic route
-
-
35704-19-9
N-(4-cyanophenyl)acetamide

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: N-(4-cyanophenyl)acetamide With sodium amide In dimethyl sulfoxide at 20 - 80℃; for 1.5h; Large scale; Stage #2: With hydrogenchloride In ethanol; dimethyl sulfoxide at 0℃; for 1.5h; pH=3 - 4; Solvent; Reagent/catalyst; Temperature; Large scale; | 97.8% |
-
-
25412-75-3
4-nitrobenzamidine

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-nitrobenzamidine With iron; acetic acid In ethanol; water at 60 - 80℃; for 4h; Stage #2: With hydrogenchloride In ethanol; water Reagent/catalyst; | 94.7% |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogenchloride / 16 h / -10 - 40 °C / Inert atmosphere 2.1: ammonia / ethanol / 2 h / 20 - 40 °C / Inert atmosphere 2.2: 0.5 h / 60 - 70 °C View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogenchloride / -10 - 40 °C / Inert atmosphere 2.1: ammonia / ethanol / 2 h / 20 - 40 °C / Inert atmosphere 2.2: 0.5 h / 60 - 70 °C View Scheme |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: C8H10N2O*2ClH With ammonia In ethanol at 20 - 40℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride In ethanol; water at 60 - 70℃; for 0.5h; Concentration; Solvent; Temperature; | 18.3 g |
| Stage #1: C8H10N2O*2ClH With ammonia In ethanol at 20 - 40℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride In ethanol; water at 60 - 70℃; for 0.5h; Concentration; | 18.3 g |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 4-aminobenzimidate dihydrochloride With ammonia In ethanol at 20 - 40℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride In ethanol; water at 60 - 70℃; for 0.5h; Concentration; Solvent; Temperature; | 22.6 g |
| Stage #1: ethyl 4-aminobenzimidate dihydrochloride With ammonia In ethanol at 20 - 40℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride In ethanol; water at 60 - 70℃; for 0.5h; | 22.6 g |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: thionyl chloride / 20 h / 20 °C / Inert atmosphere 2: ammonium carbonate / ethanol / 20 h / 20 °C / Inert atmosphere 3: acetic acid; iron / ethanol; water / 4 h / 60 - 80 °C View Scheme | |
| Multi-step reaction with 3 steps 1: thionyl chloride / 24 h / 20 °C / Inert atmosphere 2: ammonium carbonate / methanol / 16 h / 20 °C / Inert atmosphere 3: acetic acid; iron / ethanol; water / 4 h / 60 - 80 °C View Scheme |
-
-
52708-02-8
4-nitrobenzimidic acid methyl ester

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: ammonium carbonate / methanol / 16 h / 20 °C / Inert atmosphere 2: acetic acid; iron / ethanol; water / 4 h / 60 - 80 °C View Scheme |
-
-
67-56-1
methanol

-
-
38622-91-2, 36635-61-7
[(p-methylphenyl)sulfonylmethyl]isonitrile

-
-
284044-35-5
4-isopropoxy-5-ethoxy-benzaldehyde

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
883574-18-3
(4-Carbamimidoylphenylamino)-(3-ethoxy-4-isopropoxyphenyl)acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-isopropoxy-5-ethoxy-benzaldehyde; 4-aminobenzamidine dihydrochloride In methanol at 60℃; for 0.5h; Stage #2: methanol; [(p-methylphenyl)sulfonylmethyl]isonitrile; boron trifluoride diethyl etherate at 0 - 20℃; Stage #3: With ammonia; water In ethyl acetate | 100% |

-
-
6092-54-2
n-hexyl chloroformate

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
1307233-93-7
4-aminobenzamidine-N-hexylcarbamate hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: n-hexyl chloroformate; 4-aminobenzamidine dihydrochloride With sodium hydroxide In acetone at 5 - 20℃; for 0.25h; Stage #2: With hydrogenchloride In water | 97.2% |

| Conditions | Yield |
|---|---|
| Stage #1: 2-(6-((cyclopropylmethyl)carbamoyl)-2-(methoxycarbonyl)pyridin-3-yl)-4-methoxy-5-vinyl benzoic acid; 4-aminobenzamidine dihydrochloride With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In isopropyl alcohol at 0 - 20℃; for 31h; Stage #2: methanesulfonic acid In methanol; isopropyl alcohol at 0 - 20℃; for 1h; | 94% |
| Stage #1: 2-(6-((cyclopropylmethyl)carbamoyl)-2-(methoxycarbonyl)pyridin-3-yl)-4-methoxy-5-vinyl benzoic acid; 4-aminobenzamidine dihydrochloride With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In isopropyl alcohol at 20℃; for 13h; Large scale; Stage #2: methanesulfonic acid In methanol Large scale; | 93% |


| Conditions | Yield |
|---|---|
| With pyridine; dmap In N,N-dimethyl-formamide at 100℃; for 0.5h; | 88% |
-
-
1307233-94-8
N-[[2-(chloromethyl)-1-methyl-1H-benzimidazol-5-yl]carbonyl]-N-2-pyridyl-β-alanine ethyl ester

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With tetrabutylammomium bromide; potassium carbonate; sodium iodide In acetic acid butyl ester; water at 40℃; for 2h; Reagent/catalyst; Inert atmosphere; | 88% |
| With tetrabutylammomium bromide; potassium carbonate; sodium iodide In acetic acid butyl ester; water at 40℃; for 2h; Reagent/catalyst; Inert atmosphere; | 88% |
-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
501-53-1
benzyl chloroformate

-
-
147291-70-1
4-[N-(benzyloxycarbonyl)-aminoiminomethyl]aniline

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran; dichloromethane; water | 87% |
| With sodium hydroxide In tetrahydrofuran; water for 1h; Ambient temperature; | 50% |
-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzamidine dihydrochloride With hydrogenchloride; sodium nitrite In water at 0℃; for 1h; Inert atmosphere; Stage #2: With sodium azide In water for 1.83h; Inert atmosphere; | 87% |
-
-
13141-38-3
2-phenylethynylaniline

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzamidine dihydrochloride With hydrogenchloride In water at 0℃; for 0.25h; Stage #2: With sodium nitrite In water at 0℃; for 0.25h; Stage #3: 2-phenylethynylaniline Further stages; | 85% |
-
-
67-56-1
methanol

-
-
38622-91-2, 36635-61-7
[(p-methylphenyl)sulfonylmethyl]isonitrile

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
86759-23-1
3-ethoxy-4-(2-dimethylaminoethoxy)benzaldehyde

-
-
883575-39-1
(4-Carbamimidoylphenylamino)-(4-(2-dimethylaminoethoxy)-3-ethoxyphenyl)acetic acid methyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzamidine dihydrochloride; 3-ethoxy-4-(2-dimethylaminoethoxy)benzaldehyde In methanol at 60℃; for 0.5h; Stage #2: methanol; [(p-methylphenyl)sulfonylmethyl]isonitrile; boron trifluoride diethyl etherate at 0 - 20℃; for 48h; Stage #3: With ammonia; water In methanol; ethyl acetate | 83% |


| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzamidine dihydrochloride With pyridine In N,N-dimethyl-formamide for 0.25h; Stage #2: gloutaric dichloride In N,N-dimethyl-formamide for 1.25h; Product distribution / selectivity; Heating / reflux; | 80% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 0 - 10℃; for 0.333333h; | 75% |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-(6-((cyclopropylmethyl)carbamoyl)-2-((2-morpholinoethoxy)carbonyl)pyridin-3-yl)-4-methoxy-5-vinylbenzoic acid; 4-aminobenzamidine dihydrochloride With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: With hydrogenchloride | 72% |
-
-
1140899-25-7
5,8-dichloro-2,3-diacyanoquinoxaline

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 80℃; for 12h; | 71% |

| Conditions | Yield |
|---|---|
| With pyridine In N,N-dimethyl acetamide at 0 - 20℃; for 2h; Inert atmosphere; | 71% |
-
-
67036-15-1
p-nitrophenylcarbonate hexyl ester

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 20℃; | 69% |
-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
101623-70-5
acetic acid 4-nitro-phenoxycarbonyl-oxymethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide | 67% |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 20℃; | 66% |
-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With pyridine for 2h; Heating; | 65% |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 0 - 20℃; | 65% |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; | 65% |
| With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride at -1.1 - 0.3℃; for 22h; |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-(6-((cyclopropylmethyl)carbamoyl)-2-((2-methyl-1-(pivaloyloxy)propoxy)carbonyl)pyridin-3-yl)-4-methoxy-5-vinylbenzoic acid; 4-aminobenzamidine dihydrochloride With pyridine; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: With hydrogenchloride | 65% |

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water; acetone at 20℃; | 61% |

| Conditions | Yield |
|---|---|
| With sodium acetate In water at 5℃; for 1h; | 60% |
| With sodium acetate In water at 5℃; for 1h; | 52% |
| With sodium acetate In water at 5℃; |

| Conditions | Yield |
|---|---|
| With triethylamine In ethanol at 50℃; for 3h; | 60% |
-
-
2426-87-1
3-methoxy-4-(phenylmethoxy)benzaldehyde

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
121-45-9
phosphorous acid trimethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 3-methoxy-4-(phenylmethoxy)benzaldehyde; 4-aminobenzamidine dihydrochloride; phosphorous acid trimethyl ester With acetic acid at 20℃; for 48h; Stage #2: With hydrogenchloride In water at 4℃; | 58% |


-
-
2498-50-2
4-aminobenzamidine dihydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-aminobenzamidine dihydrochloride With hydrogenchloride In water at 0℃; for 0.25h; Stage #2: With sodium nitrite In water at 0℃; for 0.25h; Stage #3: 3-((4-fluorophenyl)ethynyl)aniline Further stages; | 58% |

| Conditions | Yield |
|---|---|
| Stage #1: p-benzyloxybenzaldehyde; 4-aminobenzamidine dihydrochloride With acetic acid; N-ethyl-N,N-diisopropylamine In ethanol at 20℃; Stage #2: With sodium cyanoborohydride In ethanol at 20℃; | 57% |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
2498-50-2
4-aminobenzamidine dihydrochloride

-
-
147291-56-3
tert-butyl [(4-aminophenyl)(imino)methyl]carbamate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tetrahydrofuran for 5h; | 56% |
| Stage #1: 4-aminobenzamidine dihydrochloride With sodium hydroxide In tetrahydrofuran; water at 0 - 10℃; for 0.5h; Inert atmosphere; Stage #2: di-tert-butyl dicarbonate In tetrahydrofuran; water at 0 - 10℃; for 3h; Inert atmosphere; | 56.3 g |
4-Aminobenzamidine dihydrochloride Chemical Properties
Molecular Structure of 4-Aminobenzamidine dihydrochloride (CAS NO.2498-50-2):
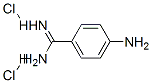
IUPAC Name: 4-aminobenzenecarboximidamide dihydrochloride
CAS: 2498-50-2
Molecular Formula: C7H11Cl2N3
Molecular Weight: 208.09
EINECS: 219-692-2
Storage temp: 2-8°C
Sensitive: Hygroscopic
BRN: 3692927
Index of Refraction: 1.629
Molar Refractivity: 37.99 cm3
Molar Volume: 106.8 cm3
Polarizability: 15.06 10-24cm3
Surface Tension: 55.2 dyne/cm
Density: 1.26 g/cm3
Flash Point: 129 °C
Enthalpy of Vaporization: 52.91 kJ/mol
Boiling Point: 289.8 °C at 760 mmHg
Vapour Pressure: 0.00216 mmHg at 25°C
Melring point: >300 °C(lit.)
Product Categories: Anilines, Aromatic Amines and Nitro Compounds;Amines; Miscellaneous; Broad Spectrum Inhibitors of Proteolytic Enzyme Classes; Other Fluorescent LabelsEnzyme Inhibitors; Protease InhibitorsFluorescent Stains; Protein Stains; Protein Stains For SpectroscopyProtease Inhibitors; Serine Protease Inhibitors; Fluorescent Labels; Fluorescent Probes, Labels, Particles and Stains; Protease Inhibitors; Imines/AmidinesFluorescent Probes, Labels, Particles and Stains; Nitrogen Compounds; Organic Building Blocks; Other Fluorescent Labels
4-Aminobenzamidine dihydrochloride Safety Profile
Hazard Codes:  Xi,
Xi, Xn
Xn
Risk Statements: 36/37/38-22 (36/37/38: Irritating to eyes, respiratory system and skin 22: Harmful if swallowed)
Safety Statements: 26-37/39-36 (26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice 37/39: Wear suitable gloves and eye/face protection 36: Wear suitable protective clothing)
WGK Germany: 3
F: 3-10-21
4-Aminobenzamidine dihydrochloride Specification
4-Aminobenzamidine dihydrochloride (CAS NO.2498-50-2) is also named as p-amino-benzamidindihydrochloride ; 4-amino-benzenecarboximidamiddihydrochloride ; 4-aminobenzenecarboximidamidedihydrochloride ; Benzamidine, p-amino-, dihydrochloride ; 4-aminobenzenecarboximidamide dihydrochloride .It is a white needle, commonly used as pharmaceutical intermediate.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View