-
Name
4-Piperidineethanol
- EINECS 210-727-7
- CAS No. 622-26-4
- Article Data14
- CAS DataBase
- Density 0.939 g/cm3
- Solubility
- Melting Point 46-47 °C(lit.)
- Formula C7H15NO
- Boiling Point 228.3 °C at 760 mmHg
- Molecular Weight 129.202
- Flash Point 101.7 °C
- Transport Information
- Appearance white crystalline low melting solid
- Safety 26-37/39-24/25
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-(4-Piperidyl)ethanol;2-(Piperidin-4-yl)-1-ethanol;2-(Piperidin-4-yl)ethanol;4-(2-Hydroxyethyl)piperidine;NSC 93818;
- PSA 32.26000
- LogP 0.69720
Synthetic route
-
-
383865-57-4
4-methoxy-7-morpholin-4-yl-benzothiazol-2-yl-amine

-
A
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| A n/a B 64% |
-
-
51052-78-9
4-piperidinylacetic acid

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With lithium aluminium tetrahydride In tetrahydrofuran at 25℃; Cooling with ice; Inert atmosphere; | 62.5% |

| Conditions | Yield |
|---|---|
| With sodium; butan-1-ol und Hydrieren des vermutlich als 2-<1,2,3,6-Tetrahydro-<4>pyridyl>-aethanol C7H13NO zu formulierenden Reaktionsprodukts in Essigsaeure an Palladium; | |
| With acetic acid; platinum Hydrogenation; |
-
-
28356-58-3
pyridine-4-acetic acid

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With platinum on activated charcoal; dimethylsulfide borane complex; hydrogen 2.) 1 atm; Multistep reaction; |

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With ethanol; sodium |
-
-
872850-91-4
2-(1,2,3,6-tetrahydro-[4]pyridyl)-ethanol

-
-
64-19-7
acetic acid

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| Hydrogenation; |

-
-
175213-46-4
1-t-Butoxycarbonyl-4-(methoxycarbonylmethyl)piperidine

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); hydrogen; sodium methylate In methanol at 100℃; under 38002.6 Torr; for 12h; Reagent/catalyst; |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
89151-44-0
4-(2-hydroxyethyl)piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In tert-butyl alcohol at 23℃; for 30h; | 100% |
| With triethylamine In chloroform at 0 - 20℃; for 1h; | 100% |
| With sodium hydroxide In water; tert-butyl alcohol at 10 - 23℃; | 100% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
162046-62-0
2-methoxy-pyridin-3-ylmethyl chloride

-
-
351410-17-8
1-[(2-methoxy-pyridin-3-yl)methyl]-4-piperidineethanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 2h; | 100% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
34619-03-9
tert-butyldicarbonate

-
-
89151-44-0
4-(2-hydroxyethyl)piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; | 100% |
| In methanol at 20℃; for 2h; Inert atmosphere; | 95% |
| In dichloromethane at 20℃; | 52 g |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
107-14-2
chloroacetonitrile

-
-
1228762-45-5
2-(4-(2-hydroxyethyl)piperidin-1-yl)acetonitrile

| Conditions | Yield |
|---|---|
| With triethylamine at 20℃; for 14h; | 100% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
1350607-38-3
2-[3-(3-chloropyrazin-2-yloxy)azetidin-1-yl]quinoline

-
-
1350603-88-1
2-(1-(3-((1-(quinolin-2-yl)azetidin-3-yl)oxy)pyrazin-2-yl)piperidin-4-yl)ethanol

| Conditions | Yield |
|---|---|
| With potassium carbonate In water; isopropyl alcohol at 160℃; for 5h; Microwave irradiation; | 100% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
98-88-4
benzoyl chloride

-
-
152902-80-2
(4-(2-hydroxyethyl)piperidin-1-yl)(phenyl)methanone

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 0℃; | 98% |
| With triethylamine In dichloromethane at 20℃; |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
108-23-6
isopropyl chloroformate

-
-
1046815-85-3
isopropyl 4-(2-hydroxyethyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| With sodium hydroxide In toluene at 20℃; for 2h; Carboxylation; | 98% |
| With triethylamine In 1,2-dimethoxyethane; toluene at 20℃; | |
| In dichloromethane; toluene at 20℃; for 2.5h; |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
1579-72-2
2,2,2-trifluoroethyl benzoate

-
-
1012802-95-7
tert-butyl 4-(2-(benzoyloxy)ethyl)piperidine-1-carboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 4-(2-Hydroxyethyl)piperidine; 2,2,2-trifluoroethyl benzoate With (μ-oxo)bis[(1,2-ethanediamino-N,N'-bis(salicylidene))iron(III)] In toluene at 120℃; for 2h; Inert atmosphere; Schlenk technique; Stage #2: di-tert-butyl dicarbonate With triethylamine In dichloromethane at 4 - 20℃; for 4h; Inert atmosphere; Schlenk technique; chemoselective reaction; | 98% |

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
1334647-42-5
5-((tert-butyl)sulfanyl)-6-chloropyrazine-2,3-dicarbonitrile

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 97% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
18162-48-6
tert-butyldimethylsilyl chloride

| Conditions | Yield |
|---|---|
| With 1H-imidazole In dichloromethane at 20℃; for 18h; | 97% |
| Stage #1: tert-butyldimethylsilyl chloride With 1H-imidazole In N,N-dimethyl-formamide at 25℃; for 1h; Stage #2: 4-(2-Hydroxyethyl)piperidine In N,N-dimethyl-formamide at 25℃; for 8h; | 82.45% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
111196-81-7
2-chloro-5-ethylpyrimidine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dimethyl sulfoxide at 110℃; | 97% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
107-31-3
Methyl formate

-
-
141047-47-4
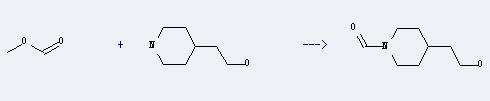
N-formyl-4-(2-hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| at 0 - 20℃; for 1h; | 95% |
| With sodium hydroxide In methanol |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
56413-95-7
2,3-dichloro-5,6-dicyanopyrazine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0℃; for 1h; Cooling with ice; | 95% |
| In tetrahydrofuran at 0℃; | 95% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
89151-44-0
4-(2-hydroxyethyl)piperidine-1-carboxylic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| In ethanol for 1h; Reflux; | 95% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
65281-95-0
5-chloro-6-(diethylamino)pyrazine-2,3-dicarbonitrile

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1.5h; | 95% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
93-58-3
benzoic acid methyl ester

-
-
1012797-96-4
2-(piperidine-4-yl)ethyl benzoate

| Conditions | Yield |
|---|---|
| With tetramethylammonium methyl carbonate In toluene for 3h; Molecular sieve; Reflux; Green chemistry; | 94% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
72966-79-1
2,6-bis(3-chloropropionamido)anthracene-9,10-dione

-
-
134888-44-1
2,6-bis<3-<4-(2-hydroxyethyl)piperidino>propionamido>anthracene-9,10-dione

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; | 93% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
93-58-3
benzoic acid methyl ester

-
A
-
1012797-96-4
2-(piperidine-4-yl)ethyl benzoate

-
B
-
1012798-08-1
N-benzoylpiperidine-4-ylethyl benzoate

| Conditions | Yield |
|---|---|
| Zn4(OCOCF3)6O In toluene for 18h; Inert atmosphere; Reflux; | A 92% B 7% |

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
1279027-08-5
tert-butyl (2-{[3-(2-chloropyrimidin-5-yl)benzyl](methyl)amino}-2-oxoethyl)carbamate

-
-
1279028-47-5
tert-butyl {2-[(3-{2-[4-(2-hydroxyethyl)piperidin-1-yl]pyrimidin-5-yl}benzyl)(methyl)amino]-2-oxoethyl}carbamate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 92% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 20℃; for 1h; Cooling with ice; | 91% |
| In tetrahydrofuran at 20℃; | 91% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
13790-39-1
6,7-dimethoxy-4-chloroquinazoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 100℃; for 16h; Sealed tube; | 91% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In butan-1-ol at 200℃; for 4h; Sealed tube; | 91% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
13790-39-1
6,7-dimethoxy-4-chloroquinazoline

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 100℃; for 16h; Sealed tube; | 91% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
93-40-3
(3,4-Dimethoxyphenyl)acetic acid

-
-
945906-57-0
2-(3,4-dimethoxyphenyl)-1-[4-(2-hydroxyethyl)piperidin-1-yl]ethanone

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 5h; | 90% |
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane at 0 - 20℃; for 5h; | 90% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine


| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 2h; | 90% |
-
-
182344-65-6
2-(6-bromo-1,3-dioxo-1H-benzo[de]isoquinolin-2(3H)-yl)-N,N-dimethylethanamine

-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In 2-methoxy-ethanol at 120℃; for 24h; Inert atmosphere; | 89% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
142873-46-9
2-bromomethyl-2,3-dihydro-2,4,6,7-tetramethyl-5-benzofuranamine

| Conditions | Yield |
|---|---|
| 88% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine

-
-
501-53-1
benzyl chloroformate

-
-
115909-91-6
1-(Benzyloxycarbonyl)-4-(2-hydroxyethyl)piperidine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In water; acetonitrile at 25℃; for 12h; Inert atmosphere; | 86% |
| With sodium carbonate In dichloromethane; water at 0 - 25℃; for 6h; Inert atmosphere; | 82% |
| With triethylamine In acetonitrile at 20℃; | 81% |
-
-
622-26-4
4-(2-Hydroxyethyl)piperidine


| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 70℃; for 4h; | 86% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In ethanol at 80℃; for 12h; Inert atmosphere; | 86% |
4-Piperidineethanol Specification
The CAS register number of 4-Piperidineethanol is 622-26-4. It also can be called as 4-(2-Hydroxyethyl)piperidine and the IUPAC name about this chemical is 2-piperidin-4-ylethanol. The molecular formula about this chemical is C7H15NO and the molecular weight is 129.20. It belongs to the Piperidine. If you want to store this chemical, please keep containers tightly closed and store it in a cool, dry, well-ventilated area away from incompatible substances.
Physical properties about 4-Piperidineethanol are: (1)ACD/LogP: -0.05; (2)ACD/LogD (pH 5.5): -3.15; (3)ACD/LogD (pH 7.4): -3.05; (4)ACD/BCF (pH 5.5): 1; (5)ACD/BCF (pH 7.4): 1; (6)ACD/KOC (pH 5.5): 1; (7)ACD/KOC (pH 7.4): 1; (8)#H bond acceptors: 2; (9)#H bond donors: 2; (10)#Freely Rotating Bonds: 3; (11)Polar Surface Area: 12.47Å2; (12)Index of Refraction: 1.454; (13)Molar Refractivity: 37.26 cm3; (14)Molar Volume: 137.5 cm3; (15)Polarizability: 14.77x10-24cm3; (16)Surface Tension: 34.6 dyne/cm; (17)Enthalpy of Vaporization: 54.04 kJ/mol; (18)Boiling Point: 228.3 °C at 760 mmHg; (19)Vapour Pressure: 0.0144 mmHg at 25°C.
Uses of 4-Piperidineethanol: it can be used to produce N-formyl-4-(2-hydroxyethyl)piperidine with formic acid methyl ester at temperature of 0 - 20 ℃. This reaction time is 1 hours. The yield is about 95%.
When you are using this chemical, please be cautious about it as the following:
This chemical is irritating to eyes, respiratory system and skin. When you are using it, wear suitable gloves and eye/face protection, you also need avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
You can still convert the following datas into molecular structure:
(1)SMILES: OCCC1CCNCC1
(2)InChI: InChI=1/C7H15NO/c9-6-3-7-1-4-8-5-2-7/h7-9H,1-6H2
(3)InChIKey: LDSQQXKSEFZAPE-UHFFFAOYAB
(4)Std. InChI: InChI=1S/C7H15NO/c9-6-3-7-1-4-8-5-2-7/h7-9H,1-6H2
(5)Std. InChIKey: LDSQQXKSEFZAPE-UHFFFAOYSA-N
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View