-
Name
5-Hydroxy-2-nitrobenzaldehyde
- EINECS 255-832-9
- CAS No. 42454-06-8
- Article Data30
- CAS DataBase
- Density 1.5 g/cm3
- Solubility
- Melting Point 165-169 °C(lit.)
- Formula C7H5NO4
- Boiling Point 373 °C at 760 mmHg
- Molecular Weight 167.121
- Flash Point 173.2 °C
- Transport Information
- Appearance
- Safety 26-36
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 2-Nitro-5-hydroxybenzaldehyde;3-Formyl-4-nitrophenol;NSC 93899;
- PSA 83.12000
- LogP 1.63610
Synthetic route
-
-
59342-81-3
ethyl (3-formyl-4-nitrophenyl) carbonate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 20℃; for 2h; Inert atmosphere; | 95% |
| With sodium hydroxide In water at 20℃; for 2h; | 80% |
| With sodium hydroxide |
-
-
454466-61-6
3-formyl-4-nitrophenyl methyl carbonate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water | 92% |
| With sodium hydroxide In water at 20℃; for 1h; | 70% |
-
-
100-83-4
meta-hydroxybenzaldehyde

-
A
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
B
-
42123-33-1
3-hydroxy-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With perchloric acid; montmorillonite K10 supported ammonium nitrate at 60℃; for 1.66667h; | A 69% B 8% |
-
-
99306-51-1
bis(3-formylphenyl) oxalate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sulfuric acid; nitric acid In methanol; (2S)-N-methyl-1-phenylpropan-2-amine hydrate; water | 66.5% |
| Stage #1: bis(3-formylphenyl) oxalate With sulfuric acid; nitric acid at -20 - -10℃; for 0.5h; Inert atmosphere; Stage #2: In water at -10 - 5℃; for 0.5h; Inert atmosphere; Stage #3: In methanol; water for 48h; Inert atmosphere; | 63% |
-
-
100-83-4
meta-hydroxybenzaldehyde

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With nitric acid | 25% |
| With nitric acid In benzene | 25% |
| With nitric acid Inert atmosphere; regioselective reaction; | 25% |
-
-
70258-76-3
carbonic acid bis-(3-formyl-4-nitro-phenyl ester)

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde
-
-
100-83-4
meta-hydroxybenzaldehyde

-
A
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
B
-
704-13-2
5-formyl-2-nitrophenol

| Conditions | Yield |
|---|---|
| With nitric acid | |
| With nitric acid |


| Conditions | Yield |
|---|---|
| at 35 - 60℃; |


-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide |
-
-
100-83-4
meta-hydroxybenzaldehyde

-
A
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With nitric acid | |
| With nitric acid |
-
-
59342-81-3
ethyl (3-formyl-4-nitrophenyl) carbonate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde
-
-
67-63-0
isopropyl alcohol

-
A
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
B
-
161518-24-7
3-(hydroxymethyl)-5-methoxy-1,2-dimethyl-1H-indole-4,7-dione

| Conditions | Yield |
|---|---|
| With dinitrogen monoxide In water Kinetics; γ-Irradiation; |

-
-
454466-57-0
3-formylphenyl methyl carbonate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 95 percent / fuming HNO3; conc. H2SO4 2: 92 percent / NaOH / H2O View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; nitric acid / 0 °C 2: sodium hydroxide / water / 1 h / 20 °C View Scheme |
-
-
68423-35-8
ethyl (3-formylphenyl) carbonate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: H2SO4, HNO3 2: aq. NaOH View Scheme | |
| Multi-step reaction with 2 steps 1: nitric acid; sulfuric acid / 4.5 h / 0 °C / Inert atmosphere 2: sodium hydroxide / water / 2 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: sulfuric acid; nitric acid / 4 h / 0 °C 2: sodium hydroxide / water / 2 h / 20 °C View Scheme |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
100-44-7
benzyl chloride

-
-
58662-54-7
5-benzyloxy-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 110℃; for 4h; | 100% |
| Stage #1: 2-nitro-5-hydroxybenzaldehyde; benzyl chloride With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; Stage #2: at 60℃; for 20h; | 95% |
| Stage #1: 2-nitro-5-hydroxybenzaldehyde; benzyl chloride With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; Stage #2: at 60℃; for 1 - 20h; | 94% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
74-88-4
methyl iodide

-
-
20357-24-8
5-methoxy-2-nitro-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In DMF (N,N-dimethyl-formamide) at 20℃; for 16h; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 23℃; for 10h; Inert atmosphere; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
-
-
110-87-2
3,4-dihydro-2H-pyran

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
272789-37-4
2-nitro-5-((tetrahydro-2H-pyran-2-yl)oxy)benzaldehyde

| Conditions | Yield |
|---|---|
| With pyridine; toluene-4-sulfonic acid In hexane; dichloromethane for 12h; | 100% |
| With pyridine; toluene-4-sulfonic acid In hexane; dichloromethane for 12h; | 100% |
| With toluene-4-sulfonic acid In diethyl ether; dichloromethane at 20℃; for 4h; Etherification; | 90% |
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; for 12h; | 84% |
| With pyridinium p-toluenesulfonate In dichloromethane at 20℃; for 16h; Etherification; | 60% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
106-96-7
propargyl bromide

-
-
478964-96-4
2-nitro-5-(prop-2-yn-1-yloxy)benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
| Stage #1: 2-nitro-5-hydroxybenzaldehyde With caesium carbonate In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: propargyl bromide In N,N-dimethyl-formamide at 20℃; for 12h; | 97% |
| Stage #1: 2-nitro-5-hydroxybenzaldehyde With potassium carbonate In N,N-dimethyl-formamide Stage #2: propargyl bromide In N,N-dimethyl-formamide for 16h; | 86% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
106-95-6
allyl bromide

-
-
717105-25-4
5-(allyloxy)-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 100% |
| With N-ethyl-N,N-diisopropylamine; sodium iodide In acetone at 20℃; for 18h; | |
| With N-ethyl-N,N-diisopropylamine at 80℃; |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
3282-30-2
pivaloyl chloride

-
-
663607-25-8
2,2-dimethyl-propionic acid 3-formyl-4-nitro-phenyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 0.5h; | 99.5% |
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 20 - 25℃; for 1h; | 75% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
77-78-1
dimethyl sulfate

-
-
20357-24-8
5-methoxy-2-nitro-benzaldehyde

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 40℃; for 5h; pH=9 - 10; | 99% |
| With potassium hydroxide | 98% |
| With alkaline solution at 40℃; | |
| With alkaline solution at 60℃; |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
75-36-5
acetyl chloride

-
-
76143-12-9
5-acetoxy-2-nitro-benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 0 - 20℃; | 99% |
| With triethylamine In acetonitrile for 1h; Ambient temperature; |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
100-39-0
benzyl bromide

-
-
58662-54-7
5-benzyloxy-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 20 - 80℃; for 2h; | 99% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 99% |
-
-
5454-83-1
5-bromovaleric acid methyl ester

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
105728-03-8
5-(3-Formyl-4-nitro-phenoxy)-pentanoic acid methyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 98% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
107-30-2
chloromethyl methyl ether

-
-
170991-12-5
2-formyl-4-(methoxymethoxy)-1-nitrobenzene

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine; dmap In dichloromethane at 20℃; | 98% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1h; | 93% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; for 1.5h; Inert atmosphere; | 85% |
| With triethylamine In dichloromethane; N,N-dimethyl acetamide; toluene at 20℃; | 73% |
| With sodium hydride In tetrahydrofuran at 20℃; for 1.33333h; |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
4296-15-5
2-iodo-1-methoxyethane

-
-
927891-86-9
5-(2-methoxyethoxy)-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 75℃; for 12h; | 98% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
54149-17-6
1-bromo-2-(2-methoxyethoxy)ethane

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; Inert atmosphere; | 98% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
60463-12-9
3-hydroxymethyl-4-nitrophenol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0 - 20℃; | 97% |
| With sodium tetrahydroborate In methanol at 0 - 20℃; for 1h; | 97% |
| With methanol; sodium tetrahydroborate at 0 - 20℃; for 3h; Inert atmosphere; | 94% |
-
-
3970-21-6
2-Methoxyethoxymethyl chloride

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
654644-18-5
5-((2-methoxy)ethoxymethoxy)-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 20℃; for 4h; | 97% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
105-53-3
diethyl malonate

| Conditions | Yield |
|---|---|
| With iron(III) chloride; N-fluorobis(benzenesulfon)imide In neat (no solvent) at 60℃; for 24h; Knoevenagel Condensation; Inert atmosphere; Green chemistry; | 97% |
-
-
2969-81-5
4-bromoethylbutanoate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
94193-35-8
ethyl 4-((3-formyl-4-nitrophenyl)oxy)butyrate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 100℃; for 1h; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 90 - 105℃; for 1.5h; | 86% |
| With potassium carbonate In N,N-dimethyl-formamide for 12h; Inert atmosphere; | 83% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide for 8h; Etherification; Heating; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 90℃; for 12h; Inert atmosphere; |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
2008-75-5
N-chloroethylpiperidine hydrochloride

-
-
485844-02-8
2-nitro-5-(2-piperidin-1-ylethoxy)benzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 4h; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 45℃; for 16h; | 85% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
3647-69-6
4-(2-chloroethyl)morpholine hydrochride

-
-
904895-36-9
5-(2-morpholinoethoxy)-2-nitrobenzaldehyde hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 2-nitro-5-hydroxybenzaldehyde; 4-(2-chloroethyl)morpholine hydrochride With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 0.666667h; Stage #2: With hydrogenchloride In methanol; ethyl acetate at 0℃; | 95% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
3619-22-5
p-toluic hydrazide

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 1h; | 95% |
-
-
25542-62-5
6-bromo-hexanoic acid ethyl ester

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
105728-04-9
6-(3-Formyl-4-nitro-phenoxy)-hexanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 94% |
-
-
29823-18-5
ethyl 7-bromoheptanoate

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
105728-05-0
7-(3-Formyl-4-nitro-phenoxy)-heptanoic acid ethyl ester

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 94% |
-
-
556-56-9
allyl iodid

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

| Conditions | Yield |
|---|---|
| With stannous fluoride In N,N-dimethyl-formamide | 94% |
-
-
294646-09-6
{2-[(pyridin-2-ylmethyl)amino]ethyl}carbamic acid tert-butyl ester

-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
1037826-16-6
(2-[(5-hydroxy-2-nitro-benzyl)-pyridin-2-ylmethyl-amino]-ethyl)-carbamic acid tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium tris(acetoxy)borohydride In 1,2-dichloro-ethane at 40℃; Inert atmosphere; Molecular sieve; | 94% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
77287-34-4, 77287-35-5, 60100-09-6
formamide

-
-
135361-38-5
N-[formamido-(5-hydroxy-2-nitrophenyl)methyl]formamide

| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane at 25℃; for 16h; | 93.4% |
| With hydrogenchloride for 12h; Ambient temperature; | 84% |
| With hydrogenchloride at 20 - 90℃; Inert atmosphere; | 73% |
| With hydrogenchloride |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
80-41-1
2-chloroethyl tosylate

-
-
116005-59-5
5-(2-chloroethoxy)-2-nitrobenzaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; | 93% |
-
-
42454-06-8
2-nitro-5-hydroxybenzaldehyde

-
-
149399-87-1
4-<(3-chloropropyl)sulfonyl>piperazine-1-carboxaldehyde

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 105℃; for 1.25h; | 93% |

| Conditions | Yield |
|---|---|
| With potassium carbonate; potassium iodide In N,N-dimethyl-formamide at 20℃; | 93% |
5-Hydroxy-2-nitrobenzaldehyde Chemical Properties
Product Name: 5-Hydroxy-2-nitrobenzaldehyde (CAS NO.42454-06-8)
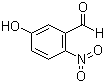
Molecular Formula: C7H5NO4
Molecular Weight: 167.12g/mol
Mol File: 42454-06-8.mol
EINECS: 255-832-9
Melting Point: 165-169 °C(lit.)
Boiling point: 373 °C at 760 mmHg
Flash Point: 173.2 °C
Density: 1.5 g/cm3
Sensitive: Air Sensitive
Index of Refraction: 1.666
Molar Refractivity: 41.43 cm3
Molar Volume: 111.3 cm3
Surface Tension: 70.5 dyne/cm
Enthalpy of Vaporization: 64.46 kJ/mol
Vapour Pressure: 4.3E-06 mmHg at 25°C
XLogP3-AA: 0.9
H-Bond Donor: 1
H-Bond Acceptor: 4
Structure Descriptors of 5-Hydroxy-2-nitrobenzaldehyde (CAS NO.42454-06-8):
IUPAC Name: 5-hydroxy-2-nitrobenzaldehyde
Canonical SMILES: C1=CC(=C(C=C1O)C=O)[N+](=O)[O-]
InChI: InChI=1S/C7H5NO4/c9-4-5-3-6(10)1-2-7(5)8(11)12/h1-4,10H
InChIKey: XLYPHUGUKGMURE-UHFFFAOYSA-N
Product Categories: Aromatic Aldehydes & Derivatives (substituted); Benzaldehyde; Aldehydes; C7; Carbonyl Compounds
5-Hydroxy-2-nitrobenzaldehyde Safety Profile
Safety Information of 5-Hydroxy-2-nitrobenzaldehyde (CAS NO.42454-06-8):
Hazard Codes: Xi
Risk Statements: 36/37/38
R36/37/38:Irritating to eyes, respiratory system and skin.
Safety Statements: 26-36
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
S36:Wear suitable protective clothing.
WGK Germany: 3
RTECS: CU6700000
5-Hydroxy-2-nitrobenzaldehyde Specification
5-Hydroxy-2-nitrobenzaldehyde , its CAS NO. is 42454-06-8, the synonyms are Benzaldehyde, 5-hydroxy-2-nitro- ; 3-Formyl-4-nitrophenol .
Related Products
- 5-Hydroxy-2-nitrobenzaldehyde
- 42457-10-3
- 4245-76-5
- 42461-84-7
- 424-64-6
- 4246-51-9
- 42465-53-2
- 42465-69-0
- 42466-59-1
- 4247-02-3
- 42471-28-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View