-
Name
5alpha-Androstane-3b,17b-diol
- EINECS 209-334-3
- CAS No. 571-20-0
- Article Data120
- CAS DataBase
- Density 1.09g/cm3
- Solubility
- Melting Point 161 °C
- Formula C19H32 O2
- Boiling Point 415°Cat760mmHg
- Molecular Weight 292.462
- Flash Point 186°C
- Transport Information
- Appearance
- Safety Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating vapors.
- Risk Codes 36/38
-
Molecular Structure
- Hazard Symbols Xi
- Synonyms 5a-Androstane-3b,17b-diol (7CI,8CI); 3b,17b-Androstanediol; 3b,17b-Dihydroxy-5a-androstane; 3b-Adiol; Maxterone; NSC 50891
- PSA 40.46000
- LogP 3.75090
Synthetic route

| Conditions | Yield |
|---|---|
| With diisobutylaluminium hydride; nickel dichloride In dichloromethane; toluene at -78℃; for 0.25h; Inert atmosphere; | 99% |
| Multi-step reaction with 2 steps 1: 97 percent / NaBH4 / methanol / 1 h / 0 - 20 °C 2: 95 percent / NaOH / methanol / 0.17 h / Heating View Scheme | |
| Multi-step reaction with 2 steps 1: sodium tetrahydroborate; methanol / 0.42 h / 20 °C / Inert atmosphere 2: potassium carbonate / methanol / 24 h / 20 °C / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In methanol at 0 - 20℃; | 95% |
| With ethanol; nickel Hydrogenation; | |
| With hydrogenchloride; acetic acid; platinum Hydrogenation.; |
-
-
3090-70-8
(3S,5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate

-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol for 0.166667h; Heating; | 95% |
| With potassium carbonate In methanol at 20℃; for 24h; Inert atmosphere; | 89% |
| With sodium hydroxide In methanol Yield given; |
-
-
1239-31-2
3β-acetoxy-5α-androstan-17-one

-
A
-
3090-70-8
(3S,5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-10,13-dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3-yl acetate

-
B
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In 1,4-dioxane; methanol for 6h; Ambient temperature; Yields of byproduct given; | A 86% B n/a |
| With sodium tetrahydroborate In 1,4-dioxane; methanol for 6h; Ambient temperature; Yields of byproduct given; | A n/a B 760 mg |
-
-
16992-89-5
3α-hydroxyandrost-4-en-17β-yl acetate

-
A
-
571-20-0
5-androgen-3,17-diol

-
B
-
1852-53-5
androstanediol

| Conditions | Yield |
|---|---|
| With sodium hydroxide; dihydrogen peroxide; diborane Product distribution; multistep reaction: 1.) THF, 0 deg C, 4 h, 2.) overnight; hydroboration of androst-4-enes; stereochemical aspects; | A n/a B n/a C 21% D 63% |
| With sodium hydroxide; dihydrogen peroxide; diborane 1.) THF, 0 deg C, 4 h, 2.) overnight; Yield given. Multistep reaction. Yields of byproduct given. Title compound not separated from byproducts; | |
| With sodium hydroxide; dihydrogen peroxide; diborane 1.) THF, 0 deg C, 4 h, 2.) overnight; Yield given. Multistep reaction. Yields of byproduct given; |

-
-
846-46-8
androstanedione

-
A
-
481-29-8
Epiandrosterone

-
B
-
53-41-8
cis-androsterone

-
C
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| for 504h; Rhodotorula mucilaginosa; | A 18% B 35% C 30% |

-
-
521-18-6
Stanolone

-
A
-
481-29-8
Epiandrosterone

-
B
-
53-41-8
cis-androsterone

-
C
-
846-46-8
androstanedione

-
D
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| for 504h; Rhodotorula mucilaginosa; | A 21% B 33% C 5% D 33% |

-
-
521-18-6
Stanolone

-
A
-
571-20-0
5-androgen-3,17-diol

-
B
-
27261-27-4
5α-androstane-3β,17β,16β-triol

-
C
-
121209-70-9
5α-androstane-2β,3α,16α,17β-tetrol

| Conditions | Yield |
|---|---|
| With fungus Gnomonia fructicola In ethanol at 24 - 26℃; for 120h; | A 3% B 20% C 3% |
| With fungus Gnomonia fructicola In ethanol at 24 - 26℃; for 120h; | A 3% B 20% C 3% |

-
-
846-46-8
androstanedione

-
A
-
481-29-8
Epiandrosterone

-
B
-
29907-31-1
11α-hydroxy-5α-androstane-3,17-dione

-
C
-
17752-36-2
3β,5α-dihydroxy-5α-androstan-17-one

-
D
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With Cephalosporium aphidicola In ethanol; dimethyl sulfoxide for 168h; | A 9% B 3.5% C 1% D 5.5% |


| Conditions | Yield |
|---|---|
| With sodium |

| Conditions | Yield |
|---|---|
| With chloroform Kochen des Reaktionsprodukts mit wss.-methanol. KOH; |
-
-
60-29-7
diethyl ether

-
-
481-29-8
Epiandrosterone

-
-
10557-57-0
propylmagnesium iodide

-
-
571-20-0
5-androgen-3,17-diol

-
-
60-29-7
diethyl ether

-
-
1239-31-2
3β-acetoxy-5α-androstan-17-one

-
-
1068-56-0
isopropylmagnesium iodide

-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| Versetzen mit wss.HCl und Erwaermen des Reaktionsprodukts mit methanol.KOH; |


| Conditions | Yield |
|---|---|
| Behandeln mit gaerender Hefe; |

| Conditions | Yield |
|---|---|
| With diethyl ether; palladium Hydrogenation; |

| Conditions | Yield |
|---|---|
| With rot causing bacteria |

| Conditions | Yield |
|---|---|
| With acetic acid; platinum Hydrogenation; | |
| With sodium tetrahydroborate; cerium(III) chloride heptahydrate In methanol Luche Cerium Reduction; |

| Conditions | Yield |
|---|---|
| bei der Einwirkung von Faeulnisbakterien; |

| Conditions | Yield |
|---|---|
| With rot causing bacteria in a(n) aqueous yeast-suspension at 36 - 37℃; | |
| With ethanol; sodium | |
| With fermenting yeast | |
| With ethanol; sodium |

| Conditions | Yield |
|---|---|
| With fermenting yeast |

| Conditions | Yield |
|---|---|
| With potassium sulfate; dipotassium peroxodisulfate; sulfuric acid; acetic acid at 25℃; Kochen des Reaktionsprodukts mit aethanol. KOH; |
-
-
1239-31-2
3β-acetoxy-5α-androstan-17-one

-
-
1068-56-0
isopropylmagnesium iodide

-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With diethyl ether Versetzen mit wss. HCl und Erhitzen des Reaktionsprodukts mit methanol. KOH; |

| Conditions | Yield |
|---|---|
| With fermenting yeast |
-
-
122747-40-4
3β-acetoxy-17β-cyclohexanecarbonyloxy-5α-androstane

-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With potassium hydroxide |

| Conditions | Yield |
|---|---|
| With sodium |
-
-
481-29-8
Epiandrosterone

-
A
-
571-20-0
5-androgen-3,17-diol

-
B
-
6247-88-7
(3β,5α,13α)-3-hydroxyandrostane-17-one

-
C
-
138313-21-0
3β-hydroxy-13,17-seco-5α-androst-13-en-17-aldehyde

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile for 0.916667h; Product distribution; Mechanism; Ambient temperature; Irradiation; other steroides; var. solvents; var. time; |

-
-
58-22-0
testosterone

-
A
-
481-29-8
Epiandrosterone

-
B
-
521-18-6
Stanolone

-
C
-
53-41-8
cis-androsterone

-
D
-
846-46-8
androstanedione

-
E
-
571-20-0
5-androgen-3,17-diol

-
F
-
1852-53-5
androstanediol

| Conditions | Yield |
|---|---|
| With total testicular homogenate of adult Sprague-Dawley rats treated with 6, des-Gly-NH210>LHRH ethylamide Product distribution; metabolism, <3H>labelled study, further: equine antibovine LH serum (JOAN-5-31-67); |

-
-
58-22-0
testosterone

-
A
-
521-18-6
Stanolone

-
B
-
53-41-8
cis-androsterone

-
C
-
846-46-8
androstanedione

-
D
-
571-20-0
5-androgen-3,17-diol

-
E
-
1852-53-5
androstanediol

| Conditions | Yield |
|---|---|
| With carbon dioxide; 5α-reductase in testicular cells of adult male Sprague-Dawley rats; oxygen; NADP at 37℃; for 1.5h; Product distribution; Kinetics; <3H>labelled, metabolism with or without 7α-hydroxytestosterone; |

-
-
58-22-0
testosterone

-
A
-
521-18-6
Stanolone

-
B
-
571-22-2
5β-androstan-17β-ol-3-one

-
C
-
571-20-0
5-androgen-3,17-diol

-
D
-
1156-92-9
4-androstenediol

-
E
-
1852-61-5
4-Androstene-3alpha,17beta-diol

| Conditions | Yield |
|---|---|
| With H2SiEt2; Rh-(R,R)-(+)-DIOP for 24h; Product distribution; other reducing agents; |


| Conditions | Yield |
|---|---|
| With potassium phosphate buffer; 3β,20β-hydroxysteroid oxidoreductase from sheep fetal blood; NADPH at 37℃; for 0.5h; Product distribution; Kinetics; Km = 74 μM, Vmax = 1.3 nmol min-1 (nmol of enzyme)-1; | |
| Multi-step reaction with 2 steps 1: 5 percent / 504 h / Rhodotorula mucilaginosa 2: 30 percent / 504 h / Rhodotorula mucilaginosa View Scheme | |
| With potassium phosphate; recombinant human aldo-keto reductase 1C1; NADPH In methanol at 37℃; pH=7; Kinetics; Reagent/catalyst; Concentration; aq. phosphate buffer; Enzymatic reaction; stereoselective reaction; | |
| With aldo-keto reductase 1D1 E120H mutant; NADPH In acetonitrile at 37℃; pH=6; Kinetics; aq. phosphate buffer; Enzymatic reaction; | |
| With potassium phosphate; rabbit aldose reductase-like protein AKR1B19; NADP In methanol at 37℃; pH=7.4; Kinetics; Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid for 0.0111667h; microwave irradiation; | 100% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite In acetic acid at 15 - 25℃; | 96% |
| at 25℃; for 25h; electrolysis: nickel net anode, cylindrical stainless steel cathode; electrolyte: 0.01M KOH/t-butanol - water (1:1); | 80% |
| In acetone at 20℃; for 48h; UV-irradiation; Inert atmosphere; | 44% |
| With tert-butyl alcohol; N-bromoacetamide | |
| With chromium(VI) oxide; acetic acid |

| Conditions | Yield |
|---|---|
| With pyridine at 28℃; for 48h; | 87% |
-
-
112-77-6
(Z)-9-octadecenoyl chloride

-
-
571-20-0
5-androgen-3,17-diol

-
-
1239669-31-8
5α-androstane-3β,17β-diol dioleate

| Conditions | Yield |
|---|---|
| With dmap; 1-butyl-3-methylimidazolium chloride In pyridine at 40℃; for 0.0166667h; Microwave irradiation; Ionic liquid; | 85% |
-
-
371-27-7
2,2,2-trifluoroethylbutyrate

-
-
571-20-0
5-androgen-3,17-diol

-
-
113999-46-5
Butyric acid (3S,5S,8R,9S,10S,13S,14S,17S)-17-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-3-yl ester

| Conditions | Yield |
|---|---|
| In acetone at 45℃; for 23h; with Chromobacterium viscosum lipase; | 83% |
-
-
571-20-0
5-androgen-3,17-diol

-
-
162465-84-1
1-(p-dimethylaminobenzoyl)-1,2,4-triazole

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane for 24h; Ambient temperature; | 77% |
-
-
571-20-0
5-androgen-3,17-diol

-
-
36239-09-5
ethyl chlorocarbonylacetate

| Conditions | Yield |
|---|---|
| With pyridine In dichloromethane at 0 - 20℃; Inert atmosphere; | 75% |
-
-
7459-33-8
linoleyl chloride

-
-
571-20-0
5-androgen-3,17-diol

-
-
1239669-32-9
5α-androstane-3β,17β-diol dilinoleate

| Conditions | Yield |
|---|---|
| With dmap; 1-butyl-3-methylimidazolium chloride In pyridine at 40℃; for 0.0166667h; Microwave irradiation; Ionic liquid; | 74% |

| Conditions | Yield |
|---|---|
| With Candida antarctica lipase B In tetrahydrofuran at 45℃; for 60h; | 71% |
-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With tetrabutylphosphonium dimethyl phosphate; 2,4,6-Triisopropylthiophenol; [Ir(2-(2,4-difluorophenyl)-4-(trifluoromethyl)pyridine)2(5,5'-bis(trifluoromethyl)-2,2'-bipyridine)]PF6 In toluene at 20℃; for 24h; Glovebox; Sealed tube; Irradiation; | 69% |
-
-
571-20-0
5-androgen-3,17-diol

-
-
185049-55-2
3-(4-tert-butylphenyl)glutaric anhydride

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In toluene Heating; | 67% |
-
-
571-20-0
5-androgen-3,17-diol

| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane for 12h; Ambient temperature; | 64% |
-
-
371-27-7
2,2,2-trifluoroethylbutyrate

-
-
571-20-0
5-androgen-3,17-diol

-
-
113999-47-6
butyric acid-(3β-hydroxy-5α-androstanyl-(17β)-ester)

| Conditions | Yield |
|---|---|
| In acetone at 45℃; for 168h; with Bacillus subtilis protease; | 60% |

| Conditions | Yield |
|---|---|
| With potato dextrose broth medium In ethanol at 24 - 26℃; for 96h; Penicillium decumbens ATCC 10436; | A 60% B 10% |
-
-
571-20-0
5-androgen-3,17-diol

-
-
149707-76-6
O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl) trichloroacetimidate

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 3h; | 36% |
-
-
571-20-0
5-androgen-3,17-diol

-
A
-
481-29-8
Epiandrosterone

-
B
-
521-18-6
Stanolone

-
C
-
846-46-8
androstanedione

| Conditions | Yield |
|---|---|
| at 25℃; for 12h; electrolysis: nickel net anode, cylindrical stainless steel cathode; electrolyte: 0.01M KOH/t-butanol - water (1:1); | A 3% B 28% C 30% |

-
-
571-20-0
5-androgen-3,17-diol

-
-
183901-63-5
2,3,4,6-tetra-O-benzoyl-α-D-mannopyranosyl trichloroacetimidate

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate In dichloromethane at 20℃; for 3h; | 15% |
-
-
571-20-0
5-androgen-3,17-diol

-
-
32212-65-0
5alpha-Androstane-3beta,11beta,17beta-triol

| Conditions | Yield |
|---|---|
| With Aspergillus tamarii KITA (QM 1223) In N,N-dimethyl-formamide at 30℃; for 120h; Microbiological reaction; | 1% |
-
-
110-86-1
pyridine

-
-
571-20-0
5-androgen-3,17-diol

-
-
75-36-5
acetyl chloride

-
-
10437-36-2
5α-androstane-3-β,17β-diol 17-acetate

5alpha-Androstane-3b,17b-diol Chemical Properties
IUPAC Name: (3S,5S,8R,9S,10S,13S,14S,17S)-10,13-dimethyl-2,3,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol
Synonyms: 10,13-Dimethylhexadecahydro-1H-cyclopenta[a]phenanthren-3,17-diol ; 10,13-Dimethylhexadecahydro-1H-cyclopenta[a]phenanthrene-3,17-diol ; 10,13-Diméthylhexadécahydro-1H-cyclopenta[a]phénanthrène-3,17-diol ; androstane-3,17-diol ; 3.alpha-17.beta.-Dihydroxy-5.alpha.-androstane ; 3alpha,17alpha-Dihydroxy-5beta-androstane ; 3alpha,17-Dihydroxy-5beta-androstane ; 3beta,17alpha-Dihydroxy-5alpha-androstane ; 3-beta,17-beta-Androstanediol ; 3beta,17beta-Dihydroxy-5alpha-androstane ; 3-beta,17-beta-Dihydroxy-5-alpha-androstane
The Molecular Formula of 5-alpha-Androstane-3-beta-17-beta-diol (571-20-0):C19H32O2
The Molecular Weight of 5-alpha-Androstane-3-beta-17-beta-diol (571-20-0):292.456180 g/mol
The Molecular Structure of 5-alpha-Androstane-3-beta-17-beta-diol (571-20-0):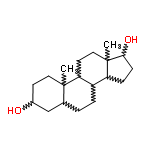
Index of Refraction: 1.546
Molar Refractivity: 84.95 cm3
Molar Volume: 268.1 cm3
Polarizability: 33.67x 10-24cm3
Surface Tension: 42.6 dyne/cm
Density: 1.09 g/cm3
Flash Point: 186 °C
Enthalpy of Vaporization: 77.17 kJ/mol
Boiling Point: 415 °C at 760 mmHg
Vapour Pressure: 1.27E-08 mmHg at 25°C
5alpha-Androstane-3b,17b-diol Uses
5-alpha-Androstane-3-beta-17-beta-diol (571- 20-0) can be used as anabolic agent.
5alpha-Androstane-3b,17b-diol Safety Profile
Experimental reproductive effects. When heated to decomposition it emits acrid smoke and irritating vapors.
Related Products
- 5alpha-Androstane-3b,17b-diol
- 571202-87-4
- 57122-94-8
- 57124-18-2
- 57124-19-3
- 57124-20-6
- 57124-87-5
- 57-12-5
- 57125-49-2
- 571-31-3
- 57132-53-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View