-
Name
6-NITROCHRYSENE
- EINECS
- CAS No. 7496-02-8
- Article Data15
- CAS DataBase
- Density 1.342g/cm3
- Solubility
-
Melting Point
220 °C (dec.)(lit.)
- Formula C18H11 N O2
- Boiling Point 505°C at 760 mmHg
- Molecular Weight 273.291
- Flash Point 252.5°C
- Transport Information
- Appearance
- Safety Confirmed carcinogen with experimental carcinogenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also CHRYSENE and NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
- Risk Codes R45; R46; R20/21/22
-
Molecular Structure
- Hazard Symbols 2134652
- Synonyms 6-Nitrochrysene;NSC 407609
- PSA 45.82000
- LogP 5.57760
Synthetic route

| Conditions | Yield |
|---|---|
| With bismuth(III) nitrate; Montmorillonite KSF for 0.25h; Nitration; | 92% |
| With sulfuric acid; nitric acid; silica gel In dichloromethane at 25℃; for 24h; Nitration; | 82% |
| With methanesulfonic acid; dinitrogen tetraoxide In dichloromethane at 20℃; for 0.25h; Product distribution; Rate constant; various additivities, nitration of polycyclic aromatic hydrocarbons by N2O4, selectivity, relative reactivities, effect of acid, base; synthetic and mechanistic study; |

| Conditions | Yield |
|---|---|
| With nitric acid In acetic anhydride at 20℃; for 120h; Yield given. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| at 100℃; | |
| at 100℃; |

-
-
218-01-9
chrysene

-
-
7664-93-9
sulfuric acid

-
-
7697-37-2
nitric acid

-
-
64-19-7
acetic acid

-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| at 40℃; | |
| at 40℃; |


| Conditions | Yield |
|---|---|
| With ammonium acetate; zinc In water Product distribution; Ambient temperature; influence of ammonium acetate; the lenght of the reducer column;; | 99% |
| With samarium; iodine In methanol for 8h; Heating; | 94% |
| With samarium; iodine In methanol for 8h; Heating; | 90% |
-
-
7496-02-8
6-nitrochrysene

-
-
114451-10-4
6-hydroxylaminochrysene

| Conditions | Yield |
|---|---|
| With 5%-palladium/activated carbon; hydrazine hydrate In tetrahydrofuran at -3℃; for 0.166667h; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| Stage #1: 6-nitrochrysene With perhydrodibenzo-18-crown-6 In N,N-dimethyl-formamide at 20℃; for 4h; Oxidation; Stage #2: acetic anhydride With pyridine Acetylation; | 65% |
-
-
7496-02-8
6-nitrochrysene

-
-
32703-90-5
12-nitro-chrysene-5,6-dione

| Conditions | Yield |
|---|---|
| With sodium dichromate; acetic acid |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| With sulfuryl dichloride; sodium acetate; nitrobenzene at 60℃; |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| With bromine; nitrobenzene at 60℃; |


| Conditions | Yield |
|---|---|
| at 100℃; |


| Conditions | Yield |
|---|---|
| at 60℃; |


| Conditions | Yield |
|---|---|
| Umsetzung des Reaktionsprodukts mit o-Phenylendiamin; |

-
-
218-01-9
chrysene

-
-
7664-93-9
sulfuric acid

-
-
7496-02-8
6-nitrochrysene

-
-
7697-37-2
nitric acid

-
-
64-19-7
acetic acid

-
-
7495-99-0
6,12-dinitro-chrysene

| Conditions | Yield |
|---|---|
| at 45 - 50℃; zuletzt bei 100grad; |

-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C 3: 72 percent / SnCl2*2H2O / ethyl acetate / 3 h / Heating 4: HgCl2; CaCO3 / acetonitrile; H2O / 12 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C 3: 72 percent / SnCl2*2H2O / ethyl acetate / 3 h / Heating 4: HgCl2; CaCO3 / acetonitrile; H2O / 12 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
625457-62-7
4-{4-[2-(bis-ethylsulfanyl-methyl)-pyrrolidine-1-carbonyl]-2-methoxy-5-nitro-phenoxy}-N-chrysen-6-yl-butyramide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
625457-64-9
4-{5-amino-4-[2-(bis-ethylsulfanyl-methyl)-pyrrolidine-1-carbonyl]-2-methoxy-phenoxy}-N-chrysen-6-yl-butyramide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C 3: 72 percent / SnCl2*2H2O / ethyl acetate / 3 h / Heating View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
625457-65-0
5-{5-Amino-4-[(S)-2-(bis-ethylsulfanyl-methyl)-pyrrolidine-1-carbonyl]-2-methoxy-phenoxy}-pentanoic acid chrysen-6-ylamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C 3: 72 percent / SnCl2*2H2O / ethyl acetate / 3 h / Heating View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
625457-63-8
5-{4-[(S)-2-(Bis-ethylsulfanyl-methyl)-pyrrolidine-1-carbonyl]-2-methoxy-5-nitro-phenoxy}-pentanoic acid chrysen-6-ylamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / SnCl2*2H2O / ethyl acetate / 3 h 2: isobutyl chloroformate; triethylamine / 12 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
219121-13-8
N-chrysen-6-yl-4-oxo-4-piperidin-1-yl-butyramide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: isobutylchloroformate; TEA / CH2Cl2 / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
219121-14-9
N-chrysen-6-yl-4-(4-methyl-piperazin-1-yl)-4-oxo-butyramide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: isobutylchloroformate; TEA / CH2Cl2 / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
347142-85-2
N-(6'-chryseneyl)-propane-3'-carboxy-1'-carboxamide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: 90 percent / TEA / CH2Cl2 / 4 h / 20 °C 3: 90 percent / NaOH / methanol / 3 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

-
-
28616-00-4
N-(6'-chryseneyl)-propane-1'-carboxamide-3'-ethylcarboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: 90 percent / TEA / CH2Cl2 / 4 h / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: isobutylchloroformate; TEA / CH2Cl2 / 20 °C 3: 80 percent / LiAlH4 / tetrahydrofuran / 12 h / Heating View Scheme |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: 90 percent / TEA / CH2Cl2 / 4 h / 20 °C 3: 90 percent / NaOH / methanol / 3 h / 20 °C 4: HOBT; BOP; N-methylmorpholine / dimethylformamide / 20 °C View Scheme |
-
-
7496-02-8
6-nitrochrysene

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 90 percent / Sm; I2 / methanol / 8 h / Heating 2: isobutylchloroformate; TEA / CH2Cl2 / 20 °C 3: 80 percent / LiAlH4 / tetrahydrofuran / 12 h / Heating View Scheme |
6-Nitrochrysene Chemical Properties
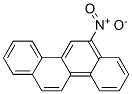
IUPAC Name: 6-nitrochrysene
Molecular Weight: 273.28544 g/mol
Molecular Formula: C18H11NO2
Density: 1.342 g/cm3
Melting Point: 220 °C (dec.)(lit.)
Boiling Point: 505 °C at 760 mmHg
Flash Point: 252.5 °C
Appreance: brown powder
Molar Volume: 203.6 cm3
Polarizability: 34.22*10-24 cm3
Surface Tension: 63.2 dyne/cm
Enthalpy of Vaporization: 74.53 kJ/mol
Vapour Pressure: 7.95E-10 mmHg at 25 °C
XLogP3: 5.5
H-Bond Acceptor: 2
Exact Mass: 273.078979
MonoIsotopic Mass: 273.078979
Topological Polar Surface Area: 43.1
Heavy Atom Count: 21
Complexity: 405
Canonical SMILES: C1=CC=C2C(=C1)C=CC3=C2C=C(C4=CC=CC=C34)[N+](=O)[O-]
InChI: InChI=1S/C18H11NO2/c20-19(21)18-11-17-13-6-2-1-5-12(13)9-10-15(17)14-7-3-4-8-16(14)18/h1-11H
InChIKey: UAWLTQJFZUYROA-UHFFFAOYSA-N
Storage temp.: 2-8 °C
Product Categories: Alphabetic; Environmental CRM; N;NA - NIApplication CRMs; Nitro-PAH; CarcinogensOrganic Building Blocks; Cancer Research; Nitro Compounds; Nitrogen Compounds
6-Nitrochrysene Toxicity Data With Reference
| 1. | mmo-sat 5 µg/plate | CNREA8 Cancer Research. 44 (1984),3408. | ||
| 2. | mma-sat 2500 ng/plate | CNREA8 Cancer Research. 44 (1984),3408. | ||
| 3. | otr-ham:emb 3700 nmol/L | CRNGDP Carcinogenesis. 4 (1983),357. |
6-Nitrochrysene Consensus Reports
NTP 10th Report on Carcinogens. IARC Cancer Review: Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 46 ,1989,p. 267.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 46 ,1989,p. 267.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human No Adequate Data IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 46 ,1989,p. 267.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) .
6-Nitrochrysene Safety Profile
Confirmed carcinogen with experimental carcinogenic data. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx. See also CHRYSENE and NITRO COMPOUNDS of AROMATIC HYDROCARBONS.
Hazard Codes:  T
T
Risk Statements: 45-46-20/21/22
R45: May cause cancer
R46: May cause heritable genetic damage
R20/21/22: Harmful by inhalation, in contact with skin and if swallowed
Safety Statements: 53-22-36/37/39-45
S53: Avoid exposure - obtain special instructions before use
S22: Do not breathe dust
S36/37/39: Wear suitable protective clothing, gloves and eye/face protection
S45: In case of accident or if you feel unwell, seek medical advice immediately (show the label whenever possible)
WGK Germany: 3
RTECS: GC1930000
6-Nitrochrysene Specification
6-Nitrochrysene (CAS NO.7496-02-8) is also called 84218, 6-Nitrochrysene (purity) ; 6-Nitrochrysene, 99% . 6-Nitrochrysene (CAS NO.7496-02-8) is chrome-red thick prismatic crystal or orange-yellow needle (recrystallized from pyridine or xylene). 6-Nitrochrysene (CAS NO.7496-02-8) reacts with tin and hydrochloric acid in glacial acetic acid. It also reacts with bromine and fuming nitric acid. When heated to decomposition 6-Nitrochrysene (CAS NO.7496-02-8) emits toxic fumes of nitrogen oxides. Flash point data for 6-Nitrochrysene (CAS NO.7496-02-8) are not available; however, It is probably combustible.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View