-
Name
9-Carboxyfluorene
- EINECS 217-866-2
- CAS No. 1989-33-9
- Article Data91
- CAS DataBase
- Density 1.309 g/cm3
- Solubility insoluble in water
- Melting Point 228-231 °C(lit.)
- Formula C14H10O2
- Boiling Point 314.9 °C at 760 mmHg
- Molecular Weight 210.232
- Flash Point 158.4 °C
- Transport Information
- Appearance white to yellow crystalline powder
- Safety 22-24/25-36/37/39-27-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Fluorene-9-carboxylicacid (6CI,7CI,8CI);9H-Fluorene-9-carboxylicacid;NSC 5322;
- PSA 37.30000
- LogP 2.88350
Synthetic route

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane; toluene | 87% |

| Conditions | Yield |
|---|---|
| With 18-crown-6 ether; potassium carbonate In dimethyl sulfoxide under 760 Torr; for 2h; Product distribution; Ambient temperature; other methylene active compounds; var. crown ethers; var. alkali carbonates; var. solvents; | 82% |
| With 18-crown-6 ether; potassium carbonate In dimethyl sulfoxide under 760 Torr; for 2h; Ambient temperature; | 82% |
| With potassium carbonate; Acetanilid In dimethyl sulfoxide at 20℃; for 4h; | 48% |

| Conditions | Yield |
|---|---|
| With samarium; chloro-trimethyl-silane; tetra-(n-butyl)ammonium iodide In acetonitrile at 20℃; under 760.051 Torr; for 2h; Electrochemical reaction; Cooling with ice; | 81% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride; nitrogen; acetic acid In water; toluene | 80.7% |
| With hydrogenchloride; potassium ethoxide In water | 72.7% |
| Stage #1: 9H-fluorene With sodium hydride In toluene at 125℃; for 4h; Inert atmosphere; Reflux; Stage #2: Diethyl carbonate In toluene at 90℃; for 4.5h; Stage #3: With hydrogenchloride; water In toluene at 20 - 40℃; | 10 g |
| Stage #1: 9H-fluorene With sodium hydride In toluene at 125℃; for 4h; Inert atmosphere; Stage #2: Diethyl carbonate In toluene at 90℃; for 4.5h; Inert atmosphere; Stage #3: With hydrogenchloride; acetic acid In water; toluene Reflux; | 10 g |
-
-
7509-44-6
10-diazophenanthren-9(10H)-one

-
A
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
B
-
84-11-7
9,10-phenanthrenequinone

| Conditions | Yield |
|---|---|
| In perchloric acid Irradiation; | A 80% B 15% |
| In sodium hydroxide Irradiation; | A 53% B 20% |
-
-
41380-42-1
9-trimethylsilyloxy-9H-fluorene-9-carbonitrile

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid; tin(ll) chloride Heating; | 74% |
-
-
76-93-7
Benzilic acid

-
A
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
B
-
467-32-3
3,3,6,6-tetraphenyl-2,5-p-dioxanedione

| Conditions | Yield |
|---|---|
| With sulfuric acid In chloroform Ambient temperature; | A 73% B 11% |

| Conditions | Yield |
|---|---|
| 69% | |
| Multi-step reaction with 3 steps 1: potassium ethylate; methanol 2: acetic acid; sulfuric acid; water 3: acetic acid; aqueous hydrogen peroxide View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether View Scheme | |
| Multi-step reaction with 2 steps 1: nBuLi 2: (i) nBuLi, THF, hexane, (ii) /BRN= 1900390/ View Scheme |

| Conditions | Yield |
|---|---|
| trifluorormethanesulfonic acid In benzene at -45℃; for 1h; | 65% |
| With aluminium trichloride; benzene zuletzt bei Siedetemperatur; | |
| With sulfuric acid In acetic acid |

| Conditions | Yield |
|---|---|
| With bis(tricyclohexylphosphine)nickel(II) dichloride; tetra-(n-butyl)ammonium iodide; zinc In N,N-dimethyl acetamide at 20℃; under 760.051 Torr; Schlenk technique; | 62% |
-
-
486-25-9
9-fluorenone

-
-
124-38-9
carbon dioxide

-
A
-
467-69-6
9-hydroxy-9-fluorene carboxylic acid

-
B
-
1689-64-1
9-Fluorenol

-
C
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With ytterbium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide under 760 Torr; for 0.833333h; Ambient temperature; | A 61% B 5% C 15% |
| With ytterbium 1.) THF, HMPA, RT, 2.) RT, 1 atm, 30 min; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With manganese; dichlorobis(trimethylphosphine)nickel In N,N-dimethyl-formamide at 50℃; under 760.051 Torr; for 24h; Schlenk technique; | 50% |
-
-
553-90-2
Dimethyl oxalate

-
-
86-73-7
9H-fluorene

-
-
917-58-8
potassium ethoxide

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With methanol Behandeln einer Loesung des Reaktionsprodukts in Essigsaeure mit wss. Schwefelsaeure bei Siedetemperatur und mit wss. Wasserstoffperoxid; |

-
-
75-44-5
phosgene

-
-
60-29-7
diethyl ether

-
-
6630-65-5
9-chlorofluorene

-
-
75-16-1
methylmagnesium bromide

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| at 0 - 5℃; anschliessende Behandlung mit festem CO2; |


| Conditions | Yield |
|---|---|
| With diethyl ether Aufgiessen der Reaktionsloesung auf festes Kohlendioxid; |

| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide at 140℃; im geschlossenen Rohr; | |
| With phosphorus; iodine; acetic acid |

| Conditions | Yield |
|---|---|
| at 160 - 170℃; im Rohr und nachfolgend Verseifen; |
-
-
86-73-7
9H-fluorene

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With potassium amide | |
| With sodium | |
| With sodium |
-
-
60-29-7
diethyl ether

-
-
3531-83-7
fluorenylsodium

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
1989-33-9
9H-fluorene-9-carboxylic acid


| Conditions | Yield |
|---|---|
| With phosphorus; hydrogen iodide | |
| With hydrogenchloride at 150 - 160℃; im Rohr; |
-
-
3531-83-7
fluorenylsodium

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
1989-33-9
9H-fluorene-9-carboxylic acid
-
-
153208-39-0
fluoren-9-yl-glyoxylic acid

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; acetic acid |

| Conditions | Yield |
|---|---|
| With diethyl ether Einleiten von Kohlendioxyd in das Reaktionsgemisch; |

| Conditions | Yield |
|---|---|
| With 1,4-dioxane; acetic acid Irradiation; |
-
-
91805-36-6
fluoren-9-ylmagnesium bromide

-
-
15719-64-9, 15719-76-3, 97762-63-5
methylammonium carbonate

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
64-19-7
acetic acid

-
-
7509-44-6
10-diazophenanthren-9(10H)-one

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| UV-Licht.Irradiation; |
-
-
40012-77-9
fluoren-9-ylidene-methanone

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| With water |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With aluminium trichloride |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Inert atmosphere; Sealed tube; | 100% |
| With acetyl chloride | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| With dmap; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In dichloromethane Inert atmosphere; Sealed tube; | 100% |
| With sulfuric acid for 8.5h; Heating; | 93% |
| With sulfuric acid | |
| With hydrogenchloride | |
| With hydrogenchloride |

| Conditions | Yield |
|---|---|
| copper(I) oxide In acetonitrile at 50℃; for 0.25h; | 100% |
| copper(I) oxide In acetonitrile at 50℃; for 0.25h; decarboxylation of arylacetic acids in the presence of Cu2O, investigation of the effect of electronwithdrawing substituents; | 100% |
| In 1-methyl-pyrrolidin-2-one at 220℃; for 0.166667h; microwave irradiation; | 90% |

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; hexane at 0 - 20℃; for 96.6667h; | 92% |
| With lithium tert-butoxide In tetrahydrofuran at 0 - 20℃; |
-
-
229-87-8
phenanthridine

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
A
-
86-73-7
9H-fluorene

-
B
-
4747-43-7
6-fluoren-9-yl-phenanthridine

-
C
-
50844-93-4
5,6,5',6'-tetrahydro-[6,6']biphenanthridinyl

| Conditions | Yield |
|---|---|
| In acetonitrile for 9h; Ambient temperature; Irradiation; | A 90% B 8% C 7% |

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
A
-
86-73-7
9H-fluorene

-
B
-
4747-43-7
6-fluoren-9-yl-phenanthridine

| Conditions | Yield |
|---|---|
| With phenanthridine In acetonitrile for 9h; Ambient temperature; Irradiation; | A 90% B 8% |
-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
-
99067-72-8
4-Iod-1-<4-nitro-phenyl>-butan

-
-
194219-13-1
9-[3-(4-Nitrophenyl)-2-propenyl]-9-fluorenecarboxylic acid

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at 0℃; for 4.91667h; | 87% |
-
-
107-10-8
propylamine

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
-
182438-56-8
N-propyl-9-fluorene-carboxamide

| Conditions | Yield |
|---|---|
| Stage #1: 9H-fluorene-9-carboxylic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 0.75h; Stage #2: propylamine In dichloromethane at -40 - 20℃; for 3h; | 87% |
-
-
7677-24-9
trimethylsilyl cyanide

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
-
21745-43-7
2-(9H-fluoren-9-yl)acetonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 9H-fluorene-9-carboxylic acid With indium(III) bromide; 1,1,3,3-Tetramethyldisiloxane; iodine In chloroform at 60℃; Inert atmosphere; Stage #2: trimethylsilyl cyanide With tetrabutyl ammonium fluoride In tetrahydrofuran; chloroform at 60℃; for 5h; Reagent/catalyst; Inert atmosphere; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| Stage #1: 9H-fluorene-9-carboxylic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 0.75h; Stage #2: benzylamine With pyridine In dichloromethane at 0 - 20℃; for 3h; | 86% |

| Conditions | Yield |
|---|---|
| Stage #1: 9H-fluorene-9-carboxylic acid With oxalyl dichloride; N,N-dimethyl-formamide In dichloromethane for 0.75h; Stage #2: ethylamine In dichloromethane at 0 - 20℃; for 3h; | 86% |
-
-
110-52-1
1,4-dibromo-butane

-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
-
182438-97-7
9-(4-bromo-butyl)-9H-fluorene-9-carboxylic acid

| Conditions | Yield |
|---|---|
| Stage #1: 9H-fluorene-9-carboxylic acid With n-butyllithium In tetrahydrofuran at 0℃; for 1h; Stage #2: 1,4-dibromo-butane In tetrahydrofuran at 0 - 20℃; for 30.5h; Further stages.; | 85% |
| With n-butyllithium In tetrahydrofuran at 0 - 20℃; for 32h; | 85% |
| Stage #1: 9H-fluorene-9-carboxylic acid With n-butyllithium In tetrahydrofuran at 0℃; for 1h; Stage #2: 1,4-dibromo-butane In tetrahydrofuran at 0 - 20℃; for 31h; | 85% |
-
-
1989-33-9
9H-fluorene-9-carboxylic acid

-
-
13500-53-3
N-benzyloxycarbonyl-(2S,4R)-4-hydroxyproline benzyl ester

-
-
1256282-36-6
(2S,4R)-dibenzyl 4-(9H-fluorene-9-carbonyloxy)pyrrolidine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane at 0 - 20℃; Inert atmosphere; | 85% |

| Conditions | Yield |
|---|---|
| 84% |
9-Carboxyfluorene Specification
The IUPAC name of this chemical is 9H-fluorene-9-carboxylic acid. With the CAS registry number 1989-33-9 and EINECS 217-866-2, it is also named as 9-Carboxyfluorene. The product's categories are Fluorene Derivatives; Benzocycles; Fluorenes, Flurenones; Organic Acids; Fluorenes; Fluorenes & Fluorenones; C13 to C42+; Carbonyl Compounds; Carboxylic Acids. It is white to yellow crystalline powder which should be sealed in the container and stored in the cool and dry place. What's more, people should ensure that the workplace has well-ventilated equipment.
The other characteristics of this product can be summarized as: (1)ACD/LogP: 2.68; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 0.87; (4)ACD/LogD (pH 7.4): -0.75; (5)ACD/BCF (pH 5.5): 1; (6)ACD/BCF (pH 7.4): 1; (7)ACD/KOC (pH 5.5): 10.52; (8)ACD/KOC (pH 7.4): 1; (9)#H bond acceptors: 2; (10)#H bond donors: 1; (11)#Freely Rotating Bonds: 1; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.668; (14)Molar Refractivity: 59.9 cm3; (15)Molar Volume: 160.5 cm3; (16)Polarizability: 23.74×10-24 cm3; (17)Surface Tension: 58.7 dyne/cm; (18)Density: 1.309 g/cm3; (19)Flash Point: 158.4 °C; (20)Enthalpy of Vaporization: 58.71 kJ/mol; (21)Boiling Point: 314.9 °C at 760 mmHg; (22)Vapour Pressure: 0.000192 mmHg at 25°C.
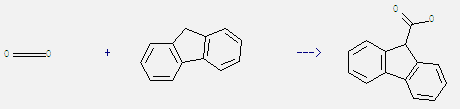
Preparation of 9H-Fluorene-9-carboxylic acid: It can be obtained by fluorene and carbon dioxide. This reaction needs reagent 18-crown-6, potassium carbonate and solvent dimethylsulfoxide at ambient temperature and pressure of 760.0002. The reaction time is 2 hours. The yield is 82%.
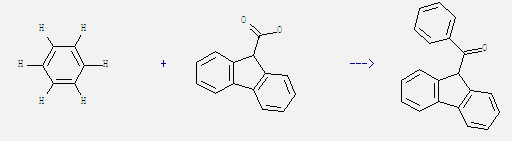
Uses of 9H-Fluorene-9-carboxylic acid: It is used in organic synthesis and as raw material of dyes, resins and pesticides. What's more, it can react with benzene to get fluoren-9-yl-phenyl ketone. This reaction needs catalytic agent TFSA at temperature of 63 °C. The reaction time is 4 hours. The yield is 60%.
When you are using this chemical, please be cautious about it as the following:
It is irritating to eyes, respiratory system and skin, so people should not breathe dust and avoid contact with skin and eyes. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. After using this chemical, take off immediately all contaminated clothing. If you want to contact this product, you must wear suitable protective clothing, gloves and eye/face protection.
People can use the following data to convert to the molecule structure.
1. SMILES:O=C(O)C3c1ccccc1c2c3cccc2
2. InChI:InChI=1/C14H10O2/c15-14(16)13-11-7-3-1-5-9(11)10-6-2-4-8-12(10)13/h1-8,13H,(H,15,16)
3. InChIKey:DNVJGJUGFFYUPT-UHFFFAOYAY
Related Products
- 9-Carboxyfluorene
- 19893-74-4
- 19893-76-6
- 19894-97-4
- 19894-99-6
- 1989-52-2
- 1989-53-3
- 198959-36-3
- 198959-37-4
- 198964-46-4
- 198967-23-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View