-
Name
Aliskiren
- EINECS 605-672-4
- CAS No. 173334-57-1
- Article Data32
- CAS DataBase
- Density 1.067 g/cm3
- Solubility
- Melting Point 98-99 °C
- Formula C30H53 N3 O6
- Boiling Point 748.4 °C at 760 mmHg
- Molecular Weight 551.767
- Flash Point 406.4 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Benzeneoctanamide,d-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-g-hydroxy-4-methoxy-3-(3-methoxypropoxy)-a,z-bis(1-methylethyl)-,(aS,gS,dS,zS)-;(2S,4S,5S,7S)-5-Amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide;Rasilez;Tekturna;
- PSA 146.13000
- LogP 5.08500
Synthetic route
-
-
173338-07-3
tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (3S,5S,6S,8S)-8-(3-amino-2,2-dimethyl-3-oxopropylcarbamoyl)-6-hydroxy-3-(4-methoxy-3-(3-methoxypropoxy)benzyl)-2,9-dimethyldecan-5-ylcarbamate With hydrogenchloride In dichloromethane at 0 - 5℃; Stage #2: With sodium hydroxide In dichloromethane pH=9; | 100% |
| With hydrogenchloride In 1,4-dioxane at 0℃; Inert atmosphere; | 99% |
| With hydrogenchloride; water In ethyl acetate at 20℃; for 0.0833333h; Flow reactor; | 96% |
-
-
1236549-06-6
(1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 10% palladium on activated charcoal; hydrogen | 97% |
| With 5%-palladium/activated carbon; hydrogen In tert-butyl methyl ether at 25℃; under 2280.15 Torr; for 16h; | 86% |
| With 5%-palladium/activated carbon; hydrogen In tert-butyl methyl ether at 25℃; under 2280.15 Torr; for 16h; | 86% |
| Stage #1: (1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester With hydrogen; acetic acid; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; Stage #2: With sodium hydroxide In ethanol pH=10; | |
| Stage #1: (1S,2S,4S)-4-(2-carbamoyl-2-methylpropyl-carbamoyl)-2-hydroxy-1-{(S)-2-[4-methoxy-3-(3-methoxypropoxy)-benzyl]-3-methylbutyl}-5-methylhexyl-carbamic acid benzyl ester With hydrogen; acetic acid; palladium 10% on activated carbon In ethanol at 20℃; under 760.051 Torr; Stage #2: With sodium hydroxide In ethanol; water pH=10; |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With hydrogen; ethanolamine; 10% palladium on activated carbon In methanol at 20℃; under 760.051 Torr; for 3h; | 87% |
-
-
324763-51-1
aminopivalinamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With 2-hydroxypyridin; hydrogenchloride; triethylamine In methanol at 80℃; | 68% |


-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Stage #1: 3-amino-2,2-dimethylpropionamide ammonium chloride; 1,1-dimethylethyl[(1S,3S)-3-[{4-methoxy-3-(3-methoxypropoxy)phenyl}methyl]-4-methyl-1-[tetrahydro-4-(1-methylethyl)-5-oxo-2-furanyl]pentyl]carbamate; sodium 2-ethylhexanoic acid at 120℃; for 1h; Stage #2: With amine HCl Stage #3: With NaA Product distribution / selectivity; | 40% |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With palladium on activated charcoal; hydrogen In methanol at 20℃; for 3h; | 38% |
-
-
324763-47-5
(2S,4S,5S,7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-5-azido-4-hydroxy-2-isopropyl-7-(4-methoxy-3-(methoxypropoxy)benzyl)-8-methylnonanamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With hydrogenchloride; hydrogen; palladium on activated charcoal In methanol | |
| With 1% Pd/C; hydrogen In ethanol | |
| In methanol | |
| With hydrogen; ethanolamine; palladium 10% on activated carbon In isopropyl alcohol for 3h; Product distribution / selectivity; | |
| With palladium 10% on activated carbon; ammonia; hydrogen In ethanol at 20℃; under 5250.53 Torr; |
-
-
666844-61-7
(2-carbamoyl-2-methylpropyl)carbamic acid benzyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: H2 / Pd()OH)2/C 2: 65 percent / 2-hydroxypyridine; Et3N 3: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-83-3
(1S,3S)-{1-formyl-3-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-4-methyl-pentyl}-carbamic acid tert-butyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Mg 2: 23 percent / H2 / Pd(OH)2/C 3: 38 percent / TPAP; NMMO 4: 65 percent / 2-hydroxypyridine; Et3N 5: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
866030-33-3
(2R,5S)-3,6-diethoxy-2-isopropyl-5-((S)-2-(4-methoxy-3-(3-methoxypropoxy)benzyl)-3-methylbutyl)-2,5-dihydropyrazine

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: HCl / acetonitrile 2: Et3N 3: NaBH4 / ethanol 4: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 5: Mg 6: 23 percent / H2 / Pd(OH)2/C 7: 38 percent / TPAP; NMMO 8: 65 percent / 2-hydroxypyridine; Et3N 9: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
866030-35-5
tert-butyl {(1S,3S)-1-((2S,4S)-4-isopropyl-5-oxotetrahydrofuran-2-yl)-3-[4-methoxy-3-(3-methoxypropoxy)benzyl]-4-methylpentyl}carbamate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / 2-hydroxypyridine; Et3N 2: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 2 steps 1.1: triethylamine; 2-hydroxypyridin / tert-butyl methyl ether / 18 h / 80 °C 2.1: trifluoroacetic acid / dichloromethane / 2 h / 20 °C 2.2: pH 10 View Scheme | |
| Multi-step reaction with 2 steps 1.1: triethylamine; 2-hydroxypyridin / 36 h / 60 - 65 °C 2.1: hydrogenchloride / dichloromethane; water / 3 h / 0 - 5 °C 2.2: 0 - 5 °C View Scheme |
-
-
866030-34-4
((1S,2S,4S)-2-hydroxy-4-hydroxymethyl-1-((S)-2-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-3-methyl-butyl)-5-methyl-hexyl)-carbamic acid tert-butyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 38 percent / TPAP; NMMO 2: 65 percent / 2-hydroxypyridine; Et3N 3: TMSCl; phenol / CH2Cl2 View Scheme |

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 23 percent / H2 / Pd(OH)2/C 2: 38 percent / TPAP; NMMO 3: 65 percent / 2-hydroxypyridine; Et3N 4: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
621-59-0
isovanillin

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 17 steps 1: 98 percent / K2CO3 / acetonitrile 2: NaBH4 / ethanol 3: PBr3 / CH2Cl2 4: 76 percent / LiHMDS / tetrahydrofuran 5: 81 percent / LiOH; H2O2 6: 95 percent / LAH / tetrahydrofuran 7: 97 percent / PPh3; NBS / CH2Cl2 8: 68 percent / n-BuLi 9: HCl / acetonitrile 10: Et3N 11: NaBH4 / ethanol 12: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 13: Mg 14: 23 percent / H2 / Pd(OH)2/C 15: 38 percent / TPAP; NMMO 16: 65 percent / 2-hydroxypyridine; Et3N 17: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
324763-51-1
aminopivalinamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 65 percent / 2-hydroxypyridine; Et3N 2: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
7505-93-3
2-cyano-2-methylpropanamide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: LAH / tetrahydrofuran 1.2: Et3N 2.1: H2 / Pd()OH)2/C 3.1: 65 percent / 2-hydroxypyridine; Et3N 4.1: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 3 steps 1: hydrogen; ammonia / raney nickel / methanol / 14 h / 40 - 45 °C / 2942.29 Torr 2: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 3: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 2 steps 1.1: hydrogen; ammonia / raney nickel / methanol / 14 h / 40 - 45 °C / 2942.29 Torr 2.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 2.2: 15 h / 85 - 90 °C 2.3: 3 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: ammonia / Raney nickel / methanol / 25 - 35 °C / Autoclave 1.2: 10 h / 60 - 65 °C / 5149.01 - 5884.58 Torr 2.1: triethylamine; 2-hydroxypyridin 3.1: hydrogenchloride / water; acetone / 20 °C 3.2: pH 8 - 9 View Scheme |
-
-
145589-03-3
(R)-4-benzyl-3-(3-methylbutyryl)oxazolidin-2-one

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 76 percent / LiHMDS / tetrahydrofuran 2: 81 percent / LiOH; H2O2 3: 95 percent / LAH / tetrahydrofuran 4: 97 percent / PPh3; NBS / CH2Cl2 5: 68 percent / n-BuLi 6: HCl / acetonitrile 7: Et3N 8: NaBH4 / ethanol 9: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 10: Mg 11: 23 percent / H2 / Pd(OH)2/C 12: 38 percent / TPAP; NMMO 13: 65 percent / 2-hydroxypyridine; Et3N 14: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 9 steps 1: 50 percent / TiCl4 / CH2Cl2 2: 82 percent / LiOH; H2O2 3: 86 percent / LAH / tetrahydrofuran 4: 61 percent / PPh3; NBS / CH2Cl2 5: Mg 6: 23 percent / H2 / Pd(OH)2/C 7: 38 percent / TPAP; NMMO 8: 65 percent / 2-hydroxypyridine; Et3N 9: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 13 steps 1.1: lithium hexamethyldisilazane / -78 °C / Inert atmosphere; Large scale 1.2: -78 °C / Inert atmosphere; Large scale 1.3: Inert atmosphere; Large scale 2.1: lithium aluminium tetrahydride / tetrahydrofuran / 3 h / 0 - 20 °C / Inert atmosphere 3.1: triethylamine / toluene / 1.5 h / 0 - 20 °C / Inert atmosphere 4.1: sodium iodide / acetonitrile / 12 h / Inert atmosphere; Reflux 5.1: lithium hydride / N,N-dimethyl acetamide / 1 h / 60 °C / Inert atmosphere 5.2: 48 h / 60 °C / Inert atmosphere 6.1: dmap; triethylamine / 3 h / 20 °C / Inert atmosphere 7.1: trifluoroacetic acid / 1 h / 20 °C / Inert atmosphere 8.1: (R)-(-)-4,12-bis(diphenylphosphino)[2.2]paracyclophane(1,5-cyclooctadiene)rhodium(I) tetrafluoroborate; hydrogen; triethylamine / methanol / 24 h / 60 °C / 22502.3 Torr / Inert atmosphere; Large scale 9.1: diphenyl phosphoryl azide; triethylamine / toluene / 1 h / 80 °C / Inert atmosphere 9.2: 8 h / Inert atmosphere; Reflux 10.1: hydrogenchloride; water / ethanol / 48 h / 70 °C / Inert atmosphere 11.1: hydrogen; palladium 10% on activated carbon / methanol / 12 h / 20 °C / Inert atmosphere 11.2: 2 h / 20 °C / Inert atmosphere 12.1: 2-Ethylhexanoic acid / n-heptane / 8 h / 70 °C / Inert atmosphere 13.1: hydrogenchloride / dichloromethane / 3 h / -10 - 0 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 14 steps 1.1: diisopropylamine; n-butyllithium / tetrahydrofuran; hexane / 2 h / -78 °C / Inert atmosphere 1.2: 20 h / -78 - 20 °C 2.1: water; dihydrogen peroxide; lithium hydroxide / tetrahydrofuran / 3 h / 0 - 20 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 6.5 h / 0 °C / Inert atmosphere; Reflux 4.1: oxalyl dichloride; dimethyl sulfoxide / dichloromethane / 1.5 h / 50 - 60 °C / Inert atmosphere 4.2: 20 °C 5.1: magnesium / tetrahydrofuran / 0.5 h / Inert atmosphere 5.2: -78 - 20 °C / Inert atmosphere 6.1: triethylamine; chlorotriethylstannane / 1,4-dioxane / 2 h / 0 °C 6.2: 20 °C 7.1: sodium periodate; ruthenium trichloride / acetonitrile / 0 - 20 °C 8.1: copper(II) sulfate / dichloromethane / 5 h / Reflux 9.1: boron trifluoride diethyl etherate / dichloromethane / 0.33 h / -78 - 20 °C 9.2: 5 h / 20 °C 10.1: diisobutylaluminium hydride / toluene / 2.5 h / -20 °C 11.1: magnesium / 0.5 h / Inert atmosphere 11.2: 3 h / -78 - 20 °C / Inert atmosphere 12.1: 10 wt% Pd(OH)2 on carbon; hydrogen / ethanol 13.1: 4-methylmorpholine N-oxide; tetrapropylammonium perruthennate / dichloromethane / 2 h / 20 °C / Inert atmosphere; Molecular sieve 14.1: triethylamine; 2-hydroxypyridin; hydrogenchloride / methanol / 80 °C View Scheme |
-
-
172900-75-3
4-methoxy-3-(3-methoxypropoxy)benzaldehyde

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 16 steps 1: NaBH4 / ethanol 2: PBr3 / CH2Cl2 3: 76 percent / LiHMDS / tetrahydrofuran 4: 81 percent / LiOH; H2O2 5: 95 percent / LAH / tetrahydrofuran 6: 97 percent / PPh3; NBS / CH2Cl2 7: 68 percent / n-BuLi 8: HCl / acetonitrile 9: Et3N 10: NaBH4 / ethanol 11: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 12: Mg 13: 23 percent / H2 / Pd(OH)2/C 14: 38 percent / TPAP; NMMO 15: 65 percent / 2-hydroxypyridine; Et3N 16: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 13 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 30 °C 1.2: pH 2 2.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 3.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 3.2: -70 - 5 °C 4.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 5.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 5.2: 5.75 h / 0 - 30 °C 6.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 7.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 7.2: 12.5 h / 0 - 5 °C 8.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 8.2: 0.5 h / 0 - 5 °C 9.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 9.2: 14.25 h / 0 - 30 °C 10.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 11.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 12.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 13.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 30 °C 1.2: pH 2 2.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 3.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 3.2: -70 - 5 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 15 °C 4.2: 11 h / 60 - 65 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 10.2: 15 h / 85 - 90 °C 10.3: 3 h View Scheme |
-
-
172900-70-8
(R)-2-[4-methoxy-3-(3-methoxypropoxy)benzyl]-3-methylbutanol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 97 percent / PPh3; NBS / CH2Cl2 2: 68 percent / n-BuLi 3: HCl / acetonitrile 4: Et3N 5: NaBH4 / ethanol 6: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 7: Mg 8: 23 percent / H2 / Pd(OH)2/C 9: 38 percent / TPAP; NMMO 10: 65 percent / 2-hydroxypyridine; Et3N 11: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 7 steps 1: 70 percent / SOCl2; pyridine 2: Mg; BrCH2CH2Br / tetrahydrofuran / 20 °C 3: H2 / [Ru2Cl4((S)-BINAP)]NEt3 / methanol / 40 h / 40 °C / 37503 Torr 4: NEt3 / CH2Cl2 5: 85 percent / NaN3; 15-crown-6; DMPU 6: 59 percent / 2-OH-pyridine; NEt3 7: H2; aq. HCl / Pd/C / methanol View Scheme | |
| Multi-step reaction with 8 steps 1.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 2.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 2.2: 12.5 h / 0 - 5 °C 3.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 3.2: 0.5 h / 0 - 5 °C 4.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 4.2: 14.25 h / 0 - 30 °C 5.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 6.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 7.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 8.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
172900-71-9
(R)-3-[4-methoxy-3-(3-methoxypropoxy)phenyl]-2-(1-methylethyl)propanoic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 12 steps 1: 95 percent / LAH / tetrahydrofuran 2: 97 percent / PPh3; NBS / CH2Cl2 3: 68 percent / n-BuLi 4: HCl / acetonitrile 5: Et3N 6: NaBH4 / ethanol 7: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 8: Mg 9: 23 percent / H2 / Pd(OH)2/C 10: 38 percent / TPAP; NMMO 11: 65 percent / 2-hydroxypyridine; Et3N 12: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 8 steps 1: 90 percent / NaBH4; I2 / 96 h / 20 °C 2: 70 percent / SOCl2; pyridine 3: Mg; BrCH2CH2Br / tetrahydrofuran / 20 °C 4: H2 / [Ru2Cl4((S)-BINAP)]NEt3 / methanol / 40 h / 40 °C / 37503 Torr 5: NEt3 / CH2Cl2 6: 85 percent / NaN3; 15-crown-6; DMPU 7: 59 percent / 2-OH-pyridine; NEt3 8: H2; aq. HCl / Pd/C / methanol View Scheme | |
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 1.2: 5.75 h / 0 - 30 °C 2.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 3.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 3.2: 12.5 h / 0 - 5 °C 4.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 4.2: 0.5 h / 0 - 5 °C 5.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 5.2: 14.25 h / 0 - 30 °C 6.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 7.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 8.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 9.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
365541-75-9
(2R)-3-methyl-2-[(phenylmethoxy)methyl]butan-1-ol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 61 percent / PPh3; NBS / CH2Cl2 2: Mg 3: 23 percent / H2 / Pd(OH)2/C 4: 38 percent / TPAP; NMMO 5: 65 percent / 2-hydroxypyridine; Et3N 6: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
365541-74-8
(4R)-3-{(2S)-3-methyl-1-oxo-2-[(phenylmethoxy)methyl]butyl}-4-(phenylmethyl)oxazolidin-2-one

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 82 percent / LiOH; H2O2 2: 86 percent / LAH / tetrahydrofuran 3: 61 percent / PPh3; NBS / CH2Cl2 4: Mg 5: 23 percent / H2 / Pd(OH)2/C 6: 38 percent / TPAP; NMMO 7: 65 percent / 2-hydroxypyridine; Et3N 8: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172901-00-7
(2S)-(benzyloxymethyl)-3-methyl-butyl bromide

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: Mg 2: 23 percent / H2 / Pd(OH)2/C 3: 38 percent / TPAP; NMMO 4: 65 percent / 2-hydroxypyridine; Et3N 5: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
656241-26-8
(2S)-3-methyl-2-[(phenylmethoxy)methyl]butanoic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 86 percent / LAH / tetrahydrofuran 2: 61 percent / PPh3; NBS / CH2Cl2 3: Mg 4: 23 percent / H2 / Pd(OH)2/C 5: 38 percent / TPAP; NMMO 6: 65 percent / 2-hydroxypyridine; Et3N 7: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-73-1
4-(bromomethyl)-1-methoxy-2-(3-methoxypropoxy)-benzene

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 14 steps 1: 76 percent / LiHMDS / tetrahydrofuran 2: 81 percent / LiOH; H2O2 3: 95 percent / LAH / tetrahydrofuran 4: 97 percent / PPh3; NBS / CH2Cl2 5: 68 percent / n-BuLi 6: HCl / acetonitrile 7: Et3N 8: NaBH4 / ethanol 9: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 10: Mg 11: 23 percent / H2 / Pd(OH)2/C 12: 38 percent / TPAP; NMMO 13: 65 percent / 2-hydroxypyridine; Et3N 14: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 11 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 1.2: -70 - 5 °C 2.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 3.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 3.2: 5.75 h / 0 - 30 °C 4.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 5.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 5.2: 12.5 h / 0 - 5 °C 6.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 6.2: 0.5 h / 0 - 5 °C 7.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 7.2: 14.25 h / 0 - 30 °C 8.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 9.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 10.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 11.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 1.2: -70 - 5 °C 2.1: sodium tetrahydroborate / tetrahydrofuran / 0 - 15 °C 2.2: 11 h / 60 - 65 °C 3.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 4.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 4.2: 12.5 h / 0 - 5 °C 5.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 5.2: 0.5 h / 0 - 5 °C 6.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 6.2: 14.25 h / 0 - 30 °C 7.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 8.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 9.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 10.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme |
-
-
172900-74-2
[4-methoxy-3-(3-methoxypropoxy)-phenyl]methanol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 15 steps 1: PBr3 / CH2Cl2 2: 76 percent / LiHMDS / tetrahydrofuran 3: 81 percent / LiOH; H2O2 4: 95 percent / LAH / tetrahydrofuran 5: 97 percent / PPh3; NBS / CH2Cl2 6: 68 percent / n-BuLi 7: HCl / acetonitrile 8: Et3N 9: NaBH4 / ethanol 10: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 11: Mg 12: 23 percent / H2 / Pd(OH)2/C 13: 38 percent / TPAP; NMMO 14: 65 percent / 2-hydroxypyridine; Et3N 15: TMSCl; phenol / CH2Cl2 View Scheme | |
| Multi-step reaction with 12 steps 1.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 2.2: -70 - 5 °C 3.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 4.2: 5.75 h / 0 - 30 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: sodium azide / ethylene glycol / 20 h / 80 - 85 °C 11.1: triethylamine; 2-hydroxypyridin / 16 h / 85 - 90 °C 12.1: hydrogen; ethanolamine / palladium 10% on activated carbon / isopropyl alcohol / 3 h View Scheme | |
| Multi-step reaction with 10 steps 1.1: phosphorus tribromide / dichloromethane / 2.17 h / 0 - 5 °C 2.1: lithium hexamethyldisilazane / tetrahydrofuran / 2 h / -70 °C / Inert atmosphere 2.2: -70 - 5 °C 3.1: dihydrogen peroxide; lithium hydroxide monohydrate / water; tetrahydrofuran / 0 - 30 °C 4.1: sodium tetrahydroborate / tetrahydrofuran / 1 h / 0 - 5 °C / Inert atmosphere 4.2: 5.75 h / 0 - 30 °C 5.1: N-chloro-succinimide; triphenylphosphine / dichloromethane / 5.5 h / -40 - 30 °C 6.1: magnesium; ethylene dibromide / tetrahydrofuran / 4.17 h / 63 - 68 °C / Inert atmosphere 6.2: 12.5 h / 0 - 5 °C 7.1: water; lithium hydroxide / tetrahydrofuran; methanol / 26 h / 65 - 70 °C 7.2: 0.5 h / 0 - 5 °C 8.1: hydrogenchloride / 1,1-dichloroethane; water / 0 - 5 °C / pH 2.5 8.2: 14.25 h / 0 - 30 °C 9.1: N-Bromosuccinimide; phosphoric acid / water; tetrahydrofuran / 1.25 h / 0 - 5 °C 10.1: 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone / 4 h / 75 - 80 °C 10.2: 15 h / 85 - 90 °C 10.3: 3 h View Scheme |
-
-
172900-69-5
(R)-4-(2-(bromomethyl)-3-methylbutyl)-1-methoxy-2-(3-methoxypropoxy)benzene

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 68 percent / n-BuLi 2: HCl / acetonitrile 3: Et3N 4: NaBH4 / ethanol 5: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 6: Mg 7: 23 percent / H2 / Pd(OH)2/C 8: 38 percent / TPAP; NMMO 9: 65 percent / 2-hydroxypyridine; Et3N 10: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-99-1
(2S,4S)-2-Amino-4-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-5-methyl-hexanoic acid ethyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: Et3N 2: NaBH4 / ethanol 3: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 4: Mg 5: 23 percent / H2 / Pd(OH)2/C 6: 38 percent / TPAP; NMMO 7: 65 percent / 2-hydroxypyridine; Et3N 8: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-82-2
(2S,4S)-2-{[(tert-butoxy)carbonyl]amino}-4-[4-methoxy-3-(3-methoxypropoxy)benzyl]-5-methylhexan-1-ol

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 2: Mg 3: 23 percent / H2 / Pd(OH)2/C 4: 38 percent / TPAP; NMMO 5: 65 percent / 2-hydroxypyridine; Et3N 6: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
172900-76-4
ethyl (2S,4S)-2-{[(tert-butoxy)carbonyl]amino}-4-[4-methoxy-3-(3-methoxypropoxy)benzyl]-5-methylhexanoate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: NaBH4 / ethanol 2: 89 percent / DMSO; ClC(O)C(O)Cl; Et3N / CH2Cl2 3: Mg 4: 23 percent / H2 / Pd(OH)2/C 5: 38 percent / TPAP; NMMO 6: 65 percent / 2-hydroxypyridine; Et3N 7: TMSCl; phenol / CH2Cl2 View Scheme |
-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In ethanol; ethyl acetate | 93.6% |
| Stage #1: (2E)-but-2-enedioic acid; (2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide In ethanol; acetonitrile at 20 - 50℃; for 0.333333h; Stage #2: In ethanol; acetonitrile at 17 - 37℃; for 12.6667h; Product distribution / selectivity; aliskiren modification A; | |
| In methanol at 40 - 45℃; Temperature; Solvent; Concentration; | 3.8 g |
-
-
144-62-7
oxalic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.333333h; | 93.36% |
-
-
110-15-6
succinic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.25h; | 92.6% |
-
-
77-92-9
citric acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In water for 0.5h; Product distribution / selectivity; | 92.37% |
-
-
110-17-8
(2E)-but-2-enedioic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
173334-58-2
(2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxypropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-(2-propan-2-yl)nonanamide hemifumarate

| Conditions | Yield |
|---|---|
| In methanol Solvent; Concentration; Time; Temperature; | 81.5% |
| In methanol at 35℃; for 1h; Product distribution / selectivity; | 74.25% |
| In ethanol at 20℃; Product distribution / selectivity; |
-
-
87-69-4
L-Tartaric acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| In ethanol; acetonitrile at 20℃; for 0.0833333h; Product distribution / selectivity; | 72.85% |
-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-75-2
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide phosphate

| Conditions | Yield |
|---|---|
| With phosphoric acid In water at 20℃; for 0.5h; Product distribution / selectivity; | 70.8% |
-
-
64-19-7
acetic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-77-4
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide acetate

| Conditions | Yield |
|---|---|
| In water for 0.5h; | 65% |
-
-
97-67-6
(S)-Malic acid

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

-
-
1327153-72-9
(2S),(4S),(5S),(7S)-N-(3-amino-2,2-dimethyl-3-oxopropyl)-2,7-di(1-methylethyl)-4-hydroxy-5-amino-8-[4-methoxy-3-(3-methoxy-propoxy)phenyl]-octanamide L-malate

| Conditions | Yield |
|---|---|
| In water for 0.5h; Product distribution / selectivity; | 63.3% |
| In ethanol; dichloromethane at 20℃; for 24h; |
-
-
838878-70-9
pentafluorophenol 4-nitroxybutyrate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 50% |
-
-
935472-60-9
4-(nitrooxy)butyl 4-nitrophenyl carbonate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 50% |
-
-
838878-70-9
pentafluorophenol 4-nitroxybutyrate

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 45% |
-
-
874446-96-5
[4-(nitrooxy)methyl]benzoic acid pentafluorophenyl ester

-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With dmap; scandium tris(trifluoromethanesulfonate) In N,N-dimethyl-formamide at 0 - 20℃; for 4h; | 30% |
-
-
173334-57-1
(2S,4S,5S,7S)-5-Amino-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxy-propoxy)-benzyl]-8-methyl-nonanoic acid (2-carbamoyl-2-methyl-propyl)-amide

| Conditions | Yield |
|---|---|
| With sulfuric acid In water; acetonitrile at 0 - 5℃; for 10 - 48h; | n/a |
| With sulfuric acid In water; acetonitrile at 0 - 5℃; for 11 - 49h; | n/a |
Aliskiren Chemical Properties
Structure of Aliskiren (CAS NO.173334-57-1):
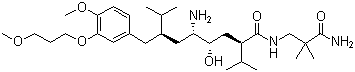
IUPAC Name: (2S,4S,5S,7S)-5-amino-N-(3-amino-2,2-dimethyl-3-oxopropyl)-4-hydroxy-7-[[4-methoxy-3-(3-methoxypropoxy)phenyl]methyl]-8-methyl-2-propan-2-ylnonanamideMolecular
Empirical Formula of Aliskiren (CAS NO.173334-57-1): C30H53N3O6
Molecular Weight: 551.7583 g/mol
H bond acceptors: 9
H bond donors: 6
Freely Rotating Bonds: 21
Index of Refraction: 1.513
Molar Refractivity: 155.53 cm3
Molar Volume: 516.9 cm3
Surface Tension: 40.5 dyne/cm
Index of Refraction: 1.513
Density: 1.067 g/cm3
Flash Point: 406.4 °C
Enthalpy of Vaporization: 114.48 kJ/mol
Boiling Point: 748.4 °C at 760 mmHg
Vapour Pressure: 1.59E-23 mmHg at 25 °C
Product Categories: Chemical Amines; Amines; Chiral Reagents; Intermediates & Fine Chemicals; Pharmaceuticals
InChI
InChI=1/C30H53N3O6/c1-19(2)22(14-21-10-11-26(38-8)27(15-21)39-13-9-12-37-7)16-24(31)25(34)17-23(20(3)4)28(35)33-18-30(5,6)29(32)36/h10-11,15,19-20,22-25,34H,9,12-14,16-18,31H2,1-8H3,(H2,32,36)(H,33,35)/t22-,23+,24-,25-/m0/s1
Smiles
C([C@@H](C[C@H](N)[C@H](C[C@@H](C(NCC(C(N)=O)(C)C)=O)C(C)C)O)C(C)C)c1ccc(OC)c(OCCCOC)c1
Aliskiren Specification
Aliskiren , its cas register number is 173334-57-1. It also can be called (2S,4S,5S,7S)-5-Amino-N-(2-carbamoyl-2-methylpropyl)-4-hydroxy-2-isopropyl-7-[4-methoxy-3-(3-methoxypropoxy)benzyl]-8-methylnonanamide and Tekturna . It is an orally active, synthetic nonpeptide renin inhibitor, used to be antihypertensive.
Related Products
- Aliskiren
- Aliskiren hemifumarate
- Aliskirenintermediate E
- 173334-58-2
- 173336-43-1
- 173336-66-8
- 173336-76-0
- 173336-82-8
- 173336-90-8
- 17333-79-8
- 173338-07-3
- 17333-84-5
- 17333-85-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View