-
Name
Aniline hydrochloride
- EINECS 205-519-8
- CAS No. 142-04-1
- Article Data180
- CAS DataBase
- Density 1.2215 g/cm3
- Solubility 1070 g/L (25 °C) in water
- Melting Point 196-198 °C(lit.)
- Formula C6H7N.HCl
- Boiling Point 184.4 °C at 760 mmHg
- Molecular Weight 129.589
- Flash Point 70 °C
- Transport Information UN 1548 6.1/PG 3
- Appearance white to off-white crystals
- Safety 26-27-36/37/39-45-61-63
- Risk Codes 23/24/25-40-41-43-48/23/24/25-50-68
-
Molecular Structure
-
Hazard Symbols
 T,
T,  N
N
- Synonyms Aniline,hydrochloride (6CI,8CI);Benzenamine, hydrochloride (9CI);Anilinium chloride;C.I. 76001;Phenylamine hydrochloride;Phenylammonium chloride;
- PSA 26.02000
- LogP 2.65200
Synthetic route

| Conditions | Yield |
|---|---|
| With hydrogenchloride; iron(III)-acetylacetonate; 1,1,3,3-Tetramethyldisiloxane In tetrahydrofuran; water at 60℃; for 24h; | 99% |
| Stage #1: nitrobenzene With iron(III) acetylacetonate; 1,1,3,3-Tetramethyldisiloxane In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; Sealed tube; Stage #2: With hydrogenchloride In tetrahydrofuran; diethyl ether for 0.0833333h; | 99% |
| Stage #1: nitrobenzene With hydrogen In ethanol at 25℃; under 11172.7 Torr; Flow reactor; Stage #2: With hydrogenchloride In ethanol Flow reactor; chemoselective reaction; | 99% |
-
-
37596-06-8
2-anilino-2-hydroxy-1,3-diphenyl-propane-1,3-dione

-
A
-
643-75-4
1,3-diphenyl-propane-1,2,3-trione

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane; water at 20℃; | A 98% B n/a |
-
-
925-90-6
ethylmagnesium bromide

-
-
103-84-4
Acetanilid

-
A
-
142-04-1
aniline hydrochloride

-
B
-
78-93-3
butanone

| Conditions | Yield |
|---|---|
| Stage #1: Acetanilid With 2-fluoropyridine; trifluoromethylsulfonic anhydride In dichloromethane at 0℃; for 0.5h; Stage #2: ethylmagnesium bromide With cerium(III) chloride In tetrahydrofuran; dichloromethane at -78℃; for 2h; Stage #3: With hydrogenchloride In ethyl acetate | A 70% B n/a |

-
-
2364-50-3
isovaleranilide

-
-
925-90-6
ethylmagnesium bromide

-
A
-
623-56-3
5-methyl-3-hexanone

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: isovaleranilide With 2-fluoropyridine; trifluoromethylsulfonic anhydride In dichloromethane at 0℃; for 0.5h; Stage #2: ethylmagnesium bromide With cerium(III) chloride In tetrahydrofuran; dichloromethane at -78℃; for 2h; Stage #3: With hydrogenchloride In ethyl acetate | A n/a B 63% |


| Conditions | Yield |
|---|---|
| With ammonium bromide; ethylenediamine at 80℃; for 5h; Microwave irradiation; | 76% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride at 20℃; for 1.5h; Cooling with ice; | 100% |
| With hydrogenchloride In acetic acid at 0.5℃; | |
| With hydrogenchloride In isopropyl ether |
-
-
25815-21-8
2-phenylimino-4,4,5,5-tetramethyl-1,3-dioxolane

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; methanol In diethyl ether at 20℃; for 1.5h; | 96% |

| Conditions | Yield |
|---|---|
| With 1-methyl-1H-imidazole; trifluoroacetic acid; palladium In ethanol |
-
-
100-00-5
4-chlorobenzonitrile

-
A
-
20265-96-7
p-chloroaniline hydrochloride

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 4-chlorobenzonitrile With hydrogen In ethanol at 25℃; under 7448.5 Torr; Flow reactor; Stage #2: With hydrogenchloride In ethanol Flow reactor; chemoselective reaction; | A 72% B n/a |
-
-
1195865-07-6
(Z)-7-benzyloxy-3-ethoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-20-3
7-benzyloxy-3-ethoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 87% B n/a |
-
-
1195865-10-1
(Z)-3-ethoxymethyl-8-methoxy-2-phenylimino-2H-chromene

-
A
-
1195865-23-6
3-ethoxymethyl-8-methoxycoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 85% B n/a |
-
-
1195865-08-7
(Z)-3-ethoxymethyl-7-methoxy-2-phenylimino-2H-chromene

-
A
-
1195865-21-4
3-ethoxymethyl-7-methoxycoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 81% B n/a |
-
-
1195865-00-9
(Z)-3-butoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-15-6
3-butoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 77% B n/a |
-
-
1195865-02-1
(Z)-6-chloro-3-ethoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-17-8
6-chloro-3-ethoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 75% B n/a |
-
-
18740-39-1
2,4-dichlorothieno[2,3-d]pyrimidine

-
A
-
142-04-1
aniline hydrochloride

-
B
-
134372-89-7
2-Chloro-4-(phenylamino)thieno[2,3-d]pyrimidine

| Conditions | Yield |
|---|---|
| With aniline In diethyl ether; ethanol | |
| With aniline In diethyl ether; ethanol |
-
-
1195865-03-2
(Z)-6-bromo-3-ethoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-18-9
6-bromo-3-ethoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 71% B n/a |
-
-
1195864-95-9
(Z)-3-methoxymethyl-2-phenylimino-2H-chromene

-
A
-
91344-68-2
3-(methoxymethyl)-2H-chromen-2-one

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 68% B n/a |
-
-
1195864-97-1
(Z)-3-ethoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-12-3
3-ethoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 73% B n/a |
-
-
1195864-98-2
(Z)-3-isopropoxymethyl-2-phenylimino-2H-chromene

-
A
-
1195865-13-4
3-isopropoxymethylcoumarin

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; water In tetrahydrofuran at 20℃; Cooling with ice; | A 73% B n/a |

| Conditions | Yield |
|---|---|
| Stage #1: N-Phenylhydroxylamine With iron(III) acetylacetonate; 1,1,3,3-Tetramethyldisiloxane In tetrahydrofuran at 60℃; for 24h; Inert atmosphere; Sealed tube; Stage #2: With hydrogenchloride In tetrahydrofuran; diethyl ether for 0.0833333h; | 77% |
-
-
17341-93-4
2,2,2-Trichloroethyl chloroformate

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; triethylamine In diethyl ether |

| Conditions | Yield |
|---|---|
| With phosphorus trichloride In dichloromethane at 0℃; for 2h; |
-
-
62-53-3
aniline

-
A
-
142-04-1
aniline hydrochloride

-
B
-
13264-13-6
hexakis(phenylamino)cyclotriphosphazene

| Conditions | Yield |
|---|---|
| With 2,2,4,4,6,6-hexachloro-1,3,5-triaza-2,4,6-triphosphorine In 1,2,4-Trimethylbenzene at 160℃; for 2h; Inert atmosphere; | A 100% B 100% |
-
-
5191-53-7
1,3,4-triphenyl-2,5-dihydro-1H-2,5-azoledione

-
A
-
4808-48-4
2,3-diphenylmaleic anhydride

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: 1,3,4-triphenyl-2,5-dihydro-1H-2,5-azoledione With potassium hydroxide In ethanol; water at 80℃; for 2h; Green chemistry; Stage #2: With hydrogenchloride In ethanol; water pH=4; Green chemistry; | A 95% B 80% |
-
-
51915-27-6
3,4,4,5-tetrachloro-4H-1,2,6-thiadiazine

-
-
62-53-3
aniline

-
C
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| In acetonitrile at 0 - 20℃; for 0.5h; | A 86% B 3% C 94% |

-
-
6833-18-7
4-methyl-N-phenylbenzamide

-
-
151-10-0
1,3-Dimethoxybenzene

-
A
-
142-04-1
aniline hydrochloride

-
B
-
78589-05-6
(2,4-dimethoxyphenyl)(p-tolyl)methanone

| Conditions | Yield |
|---|---|
| Stage #1: 4-methyl-N-phenylbenzamide With 2-fluoropyridine; trifluoromethylsulfonic anhydride In dichloromethane at -78 - 0℃; for 0.166667h; Inert atmosphere; Stage #2: 1,3-Dimethoxybenzene In dichloromethane at 0 - 20℃; Inert atmosphere; Stage #3: With hydrogenchloride; water In ethanol for 2h; Inert atmosphere; Reflux; chemoselective reaction; | A 83% B 88% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In 1,4-dioxane; water at 100℃; for 24h; | 70% |
-
-
1336-21-6
ammonium hydroxide

-
-
60-29-7
diethyl ether

-
-
61019-03-2
5-methoxy-1,3-dimethyl-2-nitrobenzene

-
A
-
102440-03-9
2,6-dimethyl-4-methoxyaniline hydrochloride

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride; zinc In dichloromethane; water; acetic acid; toluene |

-
-
650-52-2
bis(trifluoromethyl)chlorophosphine

-
A
-
348-71-0
anilino-bis-trifluoromethyl-phosphine

-
B
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With aniline In neat (no solvent) 0°C; | A 65% B n/a |
| With aniline In neat (no solvent) 0°C; | |
| With aniline In neat (no solvent) 0°C; |

| Conditions | Yield |
|---|---|
| With thionyl chloride In methanol for 4h; Reflux; | 98.5% |
-
-
151-50-8
potassium cyanide

-
-
142-04-1
aniline hydrochloride

-
-
459-57-4
4-fluorobenzaldehyde

-
-
124573-71-3
2-(4-fluorophenyl)-2-(phenylamino) acetonitrile

| Conditions | Yield |
|---|---|
| In ethanol; water 1.) 1 h cooling; 2.) 20 h room temperature; | 100% |


| Conditions | Yield |
|---|---|
| In butan-1-ol Reflux; | 100% |
-
-
862251-00-1
(R)-3-Naphthalen-1-yl-2-oxo-1-((R)-1-phenyl-ethyl)-imidazolidine-4-carboxylic acid (1R,2S,5R)-2-isopropyl-5-methyl-cyclohexyl ester

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With isopropylmagnesium chloride In tetrahydrofuran at 20℃; | 99% |

| Conditions | Yield |
|---|---|
| Stage #1: 3-Phenylpropionic acid With dmap; nicotinic anhydride In dichloromethane at 0℃; for 0.166667h; Stage #2: aniline hydrochloride In dichloromethane at 0℃; for 12h; | 97% |
| With tetrakis(2-methylimidazol-1-yl)silane In dichloromethane at 20℃; for 24h; | 71% |
-
-
862251-07-8
(R)-3-(2,4-Dimethoxy-phenyl)-2-oxo-1-((R)-1-phenyl-ethyl)-imidazolidine-4-carboxylic acid (1R,2S,5R)-2-isopropyl-5-methyl-cyclohexyl ester

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| With isopropylmagnesium chloride In tetrahydrofuran at 20℃; | 97% |
-
-
61801-32-9
methyl acetylmethanesulfonate

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| Stage #1: methyl acetylmethanesulfonate With potassium carbonate In acetone at 20℃; for 0.5h; Stage #2: aniline hydrochloride With hydrogenchloride; sodium nitrite In water; acetone at 0 - 5℃; | 97% |

| Conditions | Yield |
|---|---|
| Stage #1: C12H16N2O3 With C12H14ClNOS In N,N-dimethyl-formamide at 110℃; for 3h; Stage #2: aniline hydrochloride In N,N-dimethyl-formamide at 120℃; for 3h; | 97% |
-
-
52218-17-4
2-imino-2H-1-benzopyran-3-carboxamide

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 0.5h; Heating; | 96% |
-
-
36325-27-6, 37044-38-5, 729-30-6, 147127-97-7
4-formyl[2.2]paracyclophane

-
-
142-04-1
aniline hydrochloride

-
-
88072-30-4
(rac)-N-([2.2]paracyclophane-4-ylmethylene)aniline

| Conditions | Yield |
|---|---|
| With dichlorodiethylstannane; triethylamine In toluene Heating; | 96% |

| Conditions | Yield |
|---|---|
| With pyridine for 16h; Reflux; | 96% |

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 40℃; | 96% |
-
-
1677-46-9
4-hydroxy-1-methyl-2(1H)-quinolone

-
-
142-04-1
aniline hydrochloride

-
-
132911-19-4
1-methyl-4-(phenylamino)-2(1H)-quinolone

| Conditions | Yield |
|---|---|
| With aniline for 2.5h; Heating; | 95% |
| With aniline | 95% |
-
-
25287-28-9
N-phenyl[1-(4-methoxyphenyl)ethylidene]amine

-
-
142-04-1
aniline hydrochloride

-
-
7509-20-8
1,3,5-tris(4-methoxyphenyl)benzene

| Conditions | Yield |
|---|---|
| at 180 - 200℃; for 0.5h; | 95% |
-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| In water | 95% |
-
-
142-04-1
aniline hydrochloride

-
-
55687-43-9
methyl 4-(2-cyano-3-morpholinoallyl)-2,6-dimethoxybenzoate

-
-
55687-39-3
methyl 4-(3-anilino-2-cyanoallyl)-2,6-dimethoxybenzoate

| Conditions | Yield |
|---|---|
| In isopropyl alcohol for 0.5h; Heating; | 95% |
-
-
151-50-8
potassium cyanide

-
-
142-04-1
aniline hydrochloride

-
-
104-88-1
4-chlorobenzaldehyde

-
-
32377-36-9
anilino(4-chlorophenyl)acetonitrile

| Conditions | Yield |
|---|---|
| In ethanol; water 1.) 1 h cooling; 2.) 20 h room temperature; | 95% |

-
-
1074-12-0
phenylglyoxal hydrate

-
-
7326-64-9
2-(phenylthio)-1,3-butadiene

-
-
142-04-1
aniline hydrochloride

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 24h; aza-Diels-Alder reaction; | 95% |

Aniline hydrochloride Chemical Properties
Molecular Structure of BENZENAMINE HYDROCHLORIDE (142-04-1):
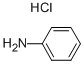
EINECS: 205-519-8
IUPAC Name: aniline hydrochloride
Molecular Formula: C6H8ClN
Molecular Weight: 129.587420 g/mol
H-Bond Donor: 2
H-Bond Acceptor: 1
SMILES: C1=CC=C(C=C1)N.Cl
InChI: InChI=1S/C6H7N.ClH/c7-6-4-2-1-3-5-6;/h1-5H,7H2;1H
InChIKey: MMCPOSDMTGQNKG-UHFFFAOYSA-N
Flash Point: 70 °C
Boiling Point: 184.4 °C at 760 mmHg
Melting Point: 196-198 °C(lit.)
Enthalpy of Vaporization: 42.44 kJ/mol
Vapour Pressure: 0.733 mmHg at 25 °C
Sensitive: Hygroscopic
Stability: Stable. Incompatible with strong oxidizing agents, strong acids.
BRN: 3593823
Aniline hydrochloride Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,65. | ||
| 2. | eye-rbt 20 mg/24H MOD | 28ZPAK Sbornik Vysledku Toxixologickeho Vysetreni Latek A Pripravku Marhold, J.V.,Institut Pro Vychovu Vedoucicn Pracovniku Chemickeho Prumyclu Praha,Czechoslovakia.: 1972,65. | ||
| 3. | sce-hmn:lym 50 µmol/L | BLFSBY Basic Life Sciences, 29b ,1984,561. | ||
| 4. | otr-rat:emb 79,500 ng/plate | JJATDK JAT, Journal of Applied Toxicology, 1 (1981),190. | ||
| 5. | sce-ham:fbr 10 µmol/L | JNCIAM Journal of the National Cancer Institute, 58 (1977),1635. | ||
| 6. | orl-rat LD50:840 mg/kg | TXAPA9 Toxicology and Applied Pharmacology, 42 (1977),417. | ||
| 7. | ipr-rat LDLo:500 mg/kg | NCNSA6 National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review, 5 (1953),11. | ||
| 8. | orl-mus LD50:841 mg/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) PB214-270 . | ||
| 9. | ipr-mus LD50:300 mg/kg | NTIS** National Technical Information Service. |
Aniline hydrochloride Consensus Reports
IARC Cancer Review: Animal Limited Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man , 27 (1982),p. 39.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . NCI Carcinogenesis Bioassay Completed; Results Positive: rat NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-130 (1978). ; Results Negative: mouse NCITR* National Cancer Institute Carcinogenesis Technical Report Series. (Bethesda, MD 20014) No. NCI-CG-TR-130 (1978). . Reported in EPA TSCA Inventory. EPA Genetic Toxicology Program.
Aniline hydrochloride Safety Profile
The safety information of BENZENAMINE HYDROCHLORIDE (142-04-1):
Hazard Codes: T ![]() ,N
,N ![]()
Risk Statements: 23/24/25-40-41-43-48/23/24/25-50-68
23/24/25: Toxic by inhalation, in contact with skin and if swallowed
40: Limited evidence of a carcinogenic effect
41: Risk of serious damage to eyes
43: May cause sensitization by skin contact
48/23/24/25: Toxic: danger of serious damage to health by prolonged exposure through inhalation, in contact with skin and if swallowed
50: Very Toxic to aquatic organisms
68: Possible risk of irreversible effects
Safety Statements: 26-27-36/37/39-45-61-63
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
27: Take off immediately all contaminated clothing
36/37/39: Wear suitable protective clothing, gloves and eye/face protection
45: In case of accident or if you feel unwell, seek medical advice immediately (show label where possible)
61: Avoid release to the environment. Refer to special instructions safety data sheet
63: In case of accident by inhalation, remove casualty to fresh air and keep at rest
RIDADR: UN 1548 6.1/PG 3
WGK Germany: 2
RTECS: CY0875000
HazardClass: 6.1
PackingGroup: III
HS Code: 29214100
F: 3-9
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Experimental teratogenic effects. Human mutation data reported. A skin and eye irritant. Combustible when exposed to heat or flame. When heated to decomposition or on contact with acid or acid fumes, it emits highly toxic fumes of aniline and chlorine compounds. Reacts explosively with aniline at 240°C/7.6 bar. Can react vigorously with oxidizing materials. To fight fire, use water, CO2, water mist or spray, dry chemical. See also ANILINE.
Aniline hydrochloride Standards and Recommendations
DOT Classification: 6.1; Label: KEEP AWAY FROM FOOD
Aniline hydrochloride Specification
BENZENAMINE HYDROCHLORIDE (142-04-1) is an aromatic ammonium salt with the appearance of white - yellow - semi-transparent flakes. It is also called ANILINE HCL ; ANILINE HYDROCHLORIDE ; Anilinechlofide ; ANILINIUM CHLORIDE ; C.I. 76001 ; benzenamine hydrochloride ; anilinechloride ; chlorhydrated’aniline , etc. BENZENAMINE HYDROCHLORIDE (142-04-1) is used in some fields such as fine chemicals, food. It is also used as reagent for analysis of carbohydrates and food. BENZENAMINE HYDROCHLORIDE (142-04-1) can be prepared by the reaction of aniline with hydrochloric acid:
C6H5NH2 + HCl → C6H5N+H3Cl-
Related Products
- Aniline
- Aniline antimonyl tartrate
- Aniline hydrochloride
- Aniline oxalate
- Aniline sulfate
- Aniline, 4,4-methylenebis(o-isopropyl-
- Aniline, N,N-diethyl-p-((p-ethylphenyl)azo)-
- Aniline, p-((4-chloro-m-tolyl)azo-N,N-dimethyl-
- aniline; butanal
- Aniline-2,4-disulfonic acid
- 14204-24-1
- 142044-38-0
- 14205-39-1
- 14205-46-0
- 14205-47-1
- 142055-86-5
- 14206-05-4
- 14206-69-0
- 142074-49-5
- 14208-10-7
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View