-
Name
ascofuranone
- EINECS
- CAS No. 38462-04-3
- Article Data1
- CAS DataBase
- Density 1.207g/cm3
- Solubility
- Melting Point
- Formula C23H29ClO5
- Boiling Point 581.2°C at 760 mmHg
- Molecular Weight 420.933
- Flash Point 305.3°C
- Transport Information
- Appearance
- Safety Moderately toxic by intraperitoneal route. See also ALDEHYDES. When heated to decomposition it emits toxic fumes of Cl−.
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms C23H29ClO5;Ascofuranone;Ascofuranon;
- PSA 83.83000
- LogP 5.22400
Synthetic route
-
-
51759-79-6
(1''S,4''S)-(-)-ascofuranol

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In dichloromethane for 3h; Ambient temperature; | 22.2% |
-
-
99529-56-3
(2E,6E,1'S,4'S)-(+)-7-(4'-hydroxy-3',3'-dimethyl-2'-oxacyclopentyl)-3,7-dimethyl-2,6-heptadienyl acetate

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1: imidazole / dimethylformamide / 6 h / Ambient temperature 2: 92.9 percent / K2CO3 / methanol; H2O / 48 h / Ambient temperature 3: 1.26 g / n-BuLi, Ph3CH, p-TsCl, LiCl / diethyl ether; hexamethylphosphoric acid triamide; hexane / 0.42 h / 0 °C 4: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 5: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 6: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 7: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 8: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 9: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-55-1, 99529-54-1, 99529-56-3, 99529-57-4
(2E,6E,1'S*,4'S*)-7-(4'-hydroxy-3',3'-dimethyl-2'-oxacyclopentyl)-3,7-dimethyl-2,6-heptadienyl acetate

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1: 5.14 g / 14 h / 80 °C 2: 76.3 percent / Et3N, HSiCl3 / benzene / 96 h / Ambient temperature 3: imidazole / dimethylformamide / 6 h / Ambient temperature 4: 92.9 percent / K2CO3 / methanol; H2O / 48 h / Ambient temperature 5: 1.26 g / n-BuLi, Ph3CH, p-TsCl, LiCl / diethyl ether; hexamethylphosphoric acid triamide; hexane / 0.42 h / 0 °C 6: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 7: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 8: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 9: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 10: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 11: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-58-4
(2E,6E,1'S,4'S)-(+)-7-(4'-t-butyldimethylsilyloxy-3',3'-dimethyl-2'-oxacyclopentyl)-3,7-dimethyl-2,6-heptadien-1-ol

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 1.26 g / n-BuLi, Ph3CH, p-TsCl, LiCl / diethyl ether; hexamethylphosphoric acid triamide; hexane / 0.42 h / 0 °C 2: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 3: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 4: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 5: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 6: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 7: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-59-5
(2E,6E,1'S,4'S)-7-(4'-t-butyldimethylsilyloxy-3',3'-dimethyl-2'-oxacyclopentyl)-3,7-dimethyl-2,6-heptadienyl chloride

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 2: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 3: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 4: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 5: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 6: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-57-3, 99529-58-5
(2E,6E,1'S,4'S)-(+)-7-(4'-t-butyldimethylsilyloxy-3',3'-dimethyl-2'-oxacyclopentyl)-3,7-dimethyl-2,6-heptadienyl acetate

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 92.9 percent / K2CO3 / methanol; H2O / 48 h / Ambient temperature 2: 1.26 g / n-BuLi, Ph3CH, p-TsCl, LiCl / diethyl ether; hexamethylphosphoric acid triamide; hexane / 0.42 h / 0 °C 3: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 4: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 5: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 6: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 7: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 8: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-60-8
(2'E,6'E,1''S,4''S)-(+)-3-<7'-(4''-t-butyldimethylsilyloxy-3'',3''-dimethyl-2''-oxacyclopentyl)-3',7'-dimethyl-2',6'-heptadienyl>-2,4-dimethoxy-6-methyl-1,4-cyclohexadiene

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 2: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 3: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 4: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 5: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-62-0
(2'E,6'E,1''S,4''S)-4-chloro-2-<7'-(4''-t-butyldimethylsilyloxy-3'',3''-dimethyl-2''-oxacyclopentyl)-3',7'-dimethyl-2',6'-heptadienyl>orcinol

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 2: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 3: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-61-9
(2'E,6'E,1''S,4''S)-3-<7'-(4''-t-butyldimethylsilyloxy-3'',3''-dimethyl-2''-oxacyclopentyl)-3',7'-dimethyl-2',6'-heptadienyl>-1,5-dichloro-6-methyl-2,4-cyclohexanedione

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 2: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 3: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 4: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-63-1
(2'E,6'E,1''S,4''S)-5-chloro-2,4-dihydroxy-6-methyl-3-<7'-(4''-t-butyldimethylsilyloxy-3'',3''-dimethyl-2''-oxacyclopentyl)-3',7'-dimethyl-2',6'-heptadienyl>benzaldehyde

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 2: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |
-
-
99477-56-2
Acetic acid (2E,6E)-7-[(2S,4S)-5,5-dimethyl-4-((R)-1-naphthalen-1-yl-ethylcarbamoyloxy)-tetrahydro-furan-2-yl]-3-methyl-octa-2,6-dienyl ester

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1: 76.3 percent / Et3N, HSiCl3 / benzene / 96 h / Ambient temperature 2: imidazole / dimethylformamide / 6 h / Ambient temperature 3: 92.9 percent / K2CO3 / methanol; H2O / 48 h / Ambient temperature 4: 1.26 g / n-BuLi, Ph3CH, p-TsCl, LiCl / diethyl ether; hexamethylphosphoric acid triamide; hexane / 0.42 h / 0 °C 5: n-BuLi, HMPA / tetrahydrofuran; hexane / 0.75 h / -70 °C 6: 50.2 percent / CaCO3, NCS / H2O; dimethylformamide / Ambient temperature 7: 57 percent / DBU / tetrahydrofuran / 6 h / Heating 8: 1) EtMgBr / 1) ether, room temp., 1 h. 10 min.; 2) ether evaporated; 100 degC, 10 min. 9: 71 percent / HF / acetonitrile; H2O / 1 h / Ambient temperature 10: 22.2 percent / pyridinium chlorochromate / CH2Cl2 / 3 h / Ambient temperature View Scheme |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; Product distribution / selectivity; | 100% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 1h; Heating / reflux; | 93% |
-
-
105-36-2
ethyl bromoacetate

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Stage #1: Ascofuranone With sodium hydride In N,N-dimethyl-formamide at 20℃; for 0.166667h; Stage #2: ethyl bromoacetate In N,N-dimethyl-formamide at 40℃; for 5.5h; | 88% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 1h; Heating; | 87% |

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 68% |
-
-
20260-53-1
pyridine-3-carbonyl chloride hydrochloride

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| With pyridine at 20℃; | 67% |
-
-
39178-35-3
isonicotinoyl chloride hydrochloride

-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| In pyridine at 20℃; for 1h; | 54% |
| Stage #1: isonicotinoyl chloride hydrochloride; Ascofuranone With pyridine at 20℃; for 1h; Stage #2: With water at 20℃; for 0.5h; | 54% |

| Conditions | Yield |
|---|---|
| With pyridine; dmap at 50℃; | 32% |
-
-
38462-04-3
Ascofuranone

-
-
1027952-60-8
3-chloro-4,6-dihydroxy-2-methyl-5-[3-methyl-7-(tetrahydro-5,5-dimethyl-4-oxo-2-furanyl)octyl]-benzaldehyde

| Conditions | Yield |
|---|---|
| With hydrogen; 5%-palladium/activated carbon In ethanol at 0℃; for 3h; | 25% |
-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| With sodium hydroxide; silver nitrate In 1,4-dioxane at 20℃; for 3h; | 24% |
| With sodium hydroxide; silver nitrate In 1,4-dioxane; water at 20℃; for 3h; | 24% |
-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| With pyridine; hydroxylamine hydrochloride at 20 - 25℃; for 3.5h; | 20% |
-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 68 percent / pyridine / 20 °C 2: 24 percent / K2CO3 / acetone / 2 h / Heating 3: 90 percent / aq. NaOH / methanol / 4 h / 20 °C View Scheme |
-
-
38462-04-3
Ascofuranone

-
-
290361-56-7
4-O-Acetyl-2-O-methyl-ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 68 percent / pyridine / 20 °C 2: 24 percent / K2CO3 / acetone / 2 h / Heating View Scheme |
-
-
38462-04-3
Ascofuranone

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: NaH / dimethylformamide / 0.17 h / 20 °C 1.2: 88 percent / dimethylformamide / 5.5 h / 40 °C 2.1: 91 percent / aq. K2CO3 / methanol / 2 h / 20 °C View Scheme |
Ascofuranone Chemical Properties
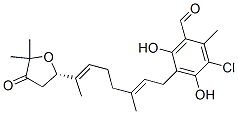
IUPAC Name: 5-chloro-3-[(2E,6E)-7-[(2S)-5,5-dimethyl-4-oxooxolan-2-yl]-3-methylocta-2,6-dienyl]-2,4-dihydroxy-6-methylbenzaldehyde
Molecular Weight: 420.92636 g/mol
Molecular Formula: C23H29ClO5
Density: 1.207 g/cm3
Boiling Point: 581.2 °C at 760 mmHg
Flash Point: 305.3 °C
Vapour Pressure: 4.18E-14 mmHg at 25°C
Index of Refraction: 1.577 Molar
Refractivity: 115.63 cm3
Molar Volume: 348.6 cm3
Polarizability: 45.84*10-24cm3
Surface Tension: 47.5 dyne/cm
Enthalpy of Vaporization: 90.13 kJ/mol
XLogP3-AA: 5.5
H-Bond Donor: 2
H-Bond Acceptor: 5
Rotatable Bond Count: 7
Tautomer Count: 50
Exact Mass: 420.170352
MonoIsotopic Mass: 420.170352
Topological Polar Surface Area: 83.8
Heavy Atom Count: 29
Complexity: 668
Defined Atom StereoCenter Count: 1
Isomeric SMILES: CC1=C(C(=C(C(=C1Cl)O)C/C=C(\C)/CC/C=C(\C)/[C@@H]2CC(=O)C(O2)(C)C)O)C=O
InChI: InChI=1S/C23H29ClO5/c1-13(7-6-8-14(2)18-11-19(26)23(4,5)29-18)9-10-16-21(27)17(12-25)15(3)20(24)22(16)28/h8-9,12,18,27-28H,6-7,10-11H2,1-5H3/b13-9+,14-8+/t18-/m0/s1
InChIKey: VGYPZLGWVQQOST-JUERRSSISA-N
Ascofuranone Uses
Ascofuranone (CAS NO.38462-04-3) is a lead compound in efforts to produce other drugs targeting this enzyme for the treatment of sleeping sickness. And it is an antibiotic produced by the fungus Ascochyta visiae that inhibits the Trypanosoma brucei alternative oxidase. The compound is effective both in infections in mice and in vitro cell culture. Ascofuranone has also been reported to have anti-tumor activity, and modulate the immune system.
Ascofuranone Toxicity Data With Reference
| 1. | ipr-rat LD50:1350 mg/kg | JANTAJ Journal of Antibiotics. 26 (1973),681. | ||
| 2. | ipr-mus LD50:2220 mg/kg | JANTAJ Journal of Antibiotics. 26 (1973),681. |
Ascofuranone Safety Profile
Moderately toxic by intraperitoneal route. See also ALDEHYDES. When heated to decomposition it emits toxic fumes of Cl−.
Ascofuranone Specification
The Ascofuranone (CAS NO.38462-04-3) is also called 2-Methyl-3-chloro-4,6-dihydroxy-5-[(2E,6E)-3-methyl-7-[(2S)-4-oxo-5,5-dimethyltetrahydrofuran-2-yl]-2,6-octadienyl]benzaldehyde ; 3-Chloro-4,6-dihydroxy-2-methyl-5-[(2E,6E)-3-methyl-7-[(S)-tetrahydro-5,5-dimethyl-4-oxofuran-2-yl]-2,6-octadienyl]benzaldehyde ; Ascofranone ; Ascofuranon . Ascofuranone (CAS NO.38462-04-3) is high toxic. It can produce toxic chloride smoke when buring. So the storage environment should be ventilate, low-temperature and dry.
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View