-
Name
Azelaic acid
- EINECS 204-669-1
- CAS No. 123-99-9
- Article Data309
- CAS DataBase
- Density 1.131 g/cm3
- Solubility warer: 2.4 g/L (20 °C)
- Melting Point 98-103 ºC
- Formula C9H16O4
- Boiling Point 370.5 ºC at 760 mmHg
- Molecular Weight 188.224
- Flash Point 192.1 ºC
- Transport Information
- Appearance white to cream solid
- Safety 24/25-36-26
- Risk Codes 36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 1, 9-Nonanedioic acid;Nonanedioic acid;Azelaic acid, technical grade;Lepargylic acid;Heptanedicarboxylic acid;Nonanedioic acid Azelaic acid;Empol 1144;1,7-Heptanedicarboxylic acid;Emerox 1144;Emerox 1110;Ammonium Hydrogen Azelate;Azelaic Acid 99%;
- PSA 74.60000
- LogP 1.88630
Synthetic route

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; potassium permanganate; N-benzyl-N,N,N-triethylammonium chloride; dihydrogen peroxide; oxygen at 95℃; for 8h; Reagent/catalyst; Concentration; Temperature; | 99.23% |
| With potassium permanganate | 80% |
| With oleate hydratase from S. maltophilia In aq. buffer at 35℃; for 2h; pH=8; Enzymatic reaction; | 21% |

| Conditions | Yield |
|---|---|
| With 1 wt% Au/Al2O3; oxygen; sodium hydroxide In water at 80℃; under 3750.38 Torr; for 4.33333h; Autoclave; Inert atmosphere; | A 86% B 99% |
| With copper(II) ferrite; oxygen In neat (no solvent) at 80℃; under 18751.9 Torr; for 5h; Reagent/catalyst; Temperature; Pressure; Autoclave; | A 57.24% B 46.65% |
| With sodium stannate; dihydrogen peroxide; tungsten(VI) oxide In water; tert-butyl alcohol at 130℃; for 4h; Sealed tube; |

| Conditions | Yield |
|---|---|
| With sodium periodate; RuCl3*2.9H2O In water at 20℃; for 8h; Sonication; | A 62% B 98% |
| With dihydrogen peroxide In tert-butyl alcohol at 85℃; for 3.5h; Catalytic behavior; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With potassium permanganate; sodium hydroxide In water at 50℃; for 8h; Reagent/catalyst; Temperature; | A 96% B 81% |
| With oxygen; ozone In water; acetone at 0℃; for 0.333333h; Reagent/catalyst; Solvent; Temperature; Flow reactor; | A 89% B 74% |
| With cetylpyridinium peroxotungstophosphate; dihydrogen peroxide In water at 80℃; for 1h; | A 86% B 82% |
-
-
14436-32-9
dec-9-enoic acid

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With sodium periodate; RuCl3*2.9H2O In water at 20℃; for 0.5h; Sonication; | 96% |
| With dihydrogen peroxide; cetyltrimethylammonim bromide; ortho-tungstic acid at 50 - 77℃; Inert atmosphere; | 76% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite; sodium chlorite; 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical In water; acetonitrile at 20℃; for 8h; Reagent/catalyst; Solvent; Temperature; | 94% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran at 23℃; for 120h; | A n/a B 2% C 92% |
| With sodium tetrahydroborate In tetrahydrofuran at 65℃; for 6h; | A n/a B 6% C 87% |

-
-
37056-34-1
8-cyano-octanoic acid

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 100℃; for 6h; | 90% |
| With sulfuric acid at 100℃; for 6h; | 90% |
| In anhydrous ethylene glycol dimethyl ether | |
| In sulfuric acid |

| Conditions | Yield |
|---|---|
| With water for 48h; Rhodococcus rhodochrous AJ270; | 88% |
| With potassium phosphate buffer; Rhodococcus sp. AJ270 at 30℃; for 48h; | 88% |

-
-
4594-78-9
cyclohexanone-2-propionitrile

-
-
2275-26-5
3-(2-oxocyclohexyl)propionic acid

-
-
7722-84-1
dihydrogen peroxide

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride; acetic acid In methanol | 88% |


| Conditions | Yield |
|---|---|
| With phosphotungstic acid; dihydrogen peroxide; cetylpyridinium bromide In water at 85℃; for 5h; Green chemistry; | A 86.5% B 87.3% |
| With tungstophosphoric acid *15.4 H2O; cetylpyridinium chloride; dihydrogen peroxide In water at 85℃; for 5h; | |
| With sodium stannate; dihydrogen peroxide; tungsten(VI) oxide In water; tert-butyl alcohol at 130℃; for 4h; Sealed tube; |

| Conditions | Yield |
|---|---|
| With oxygen; ozone In water; acetone at 0℃; Flow reactor; | A 87% B 76% |
| With dihydrogen peroxide; methyltrioctylammonium tetrakis(oxodiperoxotungsto)phos In water at 85℃; for 5h; | A 79 % Chromat. B 82 % Chromat. |

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With hydrogen In water at 200℃; under 15001.5 Torr; for 12h; | 86% |

| Conditions | Yield |
|---|---|
| With HeMaRaphos; water; toluene-4-sulfonic acid; palladium dichloride In tetrahydrofuran at 125℃; under 30003 Torr; for 24h; Autoclave; Green chemistry; regioselective reaction; | 86% |
-
-
141-22-0
Ricinoleic acid

-
A
-
123-99-9
azelaic acid

-
B
-
33796-87-1, 45023-83-4, 88930-09-0, 40165-87-5
β-hydroxynonanoic acid

| Conditions | Yield |
|---|---|
| With oxygen; ozone In water; acetone at 0℃; Flow reactor; | A 86% B 70% |
-
-
81212-19-3
(Z)-12-hydroxyoctadec-9-enoic acid

-
A
-
123-99-9
azelaic acid

-
B
-
33796-87-1, 45023-83-4, 88930-09-0, 40165-87-5
β-hydroxynonanoic acid

-
C
-
47244-76-8, 118201-32-4
12-hydroxy-9,10-epoxyoctadecanoic acid

| Conditions | Yield |
|---|---|
| With cetylpyridinium peroxotungstophosphate; dihydrogen peroxide In water at 80℃; for 1h; | A 84% B 84% C 4% |


| Conditions | Yield |
|---|---|
| With ozone In water; acetonitrile at 0℃; | A 84% B 83% |

| Conditions | Yield |
|---|---|
| With phosphotungstic acid; dihydrogen peroxide; cetylpyridinium bromide In water at 85℃; for 5h; Green chemistry; | A 83.2% B 60.8% |
| With tungstophosphoric acid *15.4 H2O; cetylpyridinium chloride; dihydrogen peroxide In water at 85℃; for 5h; |

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; cetyltrimethylammonim bromide; ortho-tungstic acid In water at 50 - 77℃; for 16h; Inert atmosphere; | 79% |

| Conditions | Yield |
|---|---|
| With sodium periodate; ruthenium trichloride In water; ethyl acetate; acetonitrile for 2h; | 75% |
-
-
19835-69-9
cyclooct-4-enecarboxaldehyde

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water at 330 - 350℃; for 6h; autoclave; | 73.5% |
-
-
101171-32-8
methyl 10-acetoxy-9-oxodecanoate

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With oxygen; sodium t-butanolate In diethyl ether; water for 4h; Ambient temperature; | 70% |

| Conditions | Yield |
|---|---|
| In water | 70% |

-
-
112-80-1
cis-Octadecenoic acid

-
A
-
123-99-9
azelaic acid

-
B
-
2243-24-5
N-hydroxynonan-1-imine

-
C
-
112-05-0
nonanoic acid

| Conditions | Yield |
|---|---|
| Stage #1: cis-Octadecenoic acid With oxygen; ozone at 0 - 20℃; Stage #2: With hydroxylamine hydrochloride In methanol at 0℃; Solvent; Reagent/catalyst; | A 66% B 45% C 46% D 26% |


| Conditions | Yield |
|---|---|
| With HeMaRaphos; water; toluene-4-sulfonic acid; palladium dichloride In tetrahydrofuran at 125℃; under 30003 Torr; for 24h; Autoclave; Green chemistry; regioselective reaction; | 65% |

| Conditions | Yield |
|---|---|
| With sodium hypochlorite at 20℃; for 5h; Reagent/catalyst; | 50% |

-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With methanesulfonic acid In water at 125℃; for 4h; Inert atmosphere; | 40% |
| at 120 - 130℃; for 16h; Temperature; | 11 g |
| With trimethylamine In toluene for 12h; Dean-Stark; Inert atmosphere; Reflux; Industrial scale; | 3406 kg |

| Conditions | Yield |
|---|---|
| With boron trifluoride at 65℃; for 0.333333h; | 100% |
| With sulfuric acid In dichloromethane Dean-Stark; Heating; | 95% |
| With sulfuric acid | 80% |

| Conditions | Yield |
|---|---|
| With thionyl chloride at 80℃; for 36h; Inert atmosphere; | 100% |
| With thionyl chloride at 80℃; for 24h; | 96% |
| With thionyl chloride at 20℃; | 96% |

| Conditions | Yield |
|---|---|
| With sulfuric acid; mercury(II) diacetate at 20 - 50℃; for 4h; | 100% |
-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With magnesium hydroxide; water for 0.583333h; Milling; | 100% |
-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With water; potassium hydroxide for 0.166667h; Milling; | 100% |
-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With water; sodium hydroxide for 0.166667h; Milling; | 100% |
-
-
123-99-9
azelaic acid

| Conditions | Yield |
|---|---|
| With magnesium hydroxide; water for 0.25h; Milling; | 100% |

| Conditions | Yield |
|---|---|
| With [HSO3-pmim]+[HSO4]-catalyst for 0.333333h; Reagent/catalyst; Microwave irradiation; | 99.1% |
| In 5,5-dimethyl-1,3-cyclohexadiene at 160℃; for 2h; | 94% |
-
-
123-99-9
azelaic acid

-
-
459142-93-9
1,1'-bis(4-pyridinyl)ferrocene

| Conditions | Yield |
|---|---|
| In methanol 1:1 mixt. ground for 5 min, dissolved in methanol; crystd.; | 99% |

| Conditions | Yield |
|---|---|
| With bromine; phosphorus tribromide at 80℃; for 8h; Temperature; Time; | 98% |

| Conditions | Yield |
|---|---|
| Stage #1: azelaic acid With thionyl chloride at 75℃; for 1.5h; Sealed tube; Stage #2: With bromine for 12h; Irradiation; Stage #3: methanol for 1.5h; Cooling with ice; | 98% |
| (i) SOCl2, (ii) Br2, I2, (iii) /BRN= 1098229/; Multistep reaction; | |
| (i) SOCl2, (ii) Br2, (iii) /BRN= 1098229/; Multistep reaction; |

| Conditions | Yield |
|---|---|
| tetrachlorobis(tetrahydrofuran)hafnium(IV) In o-xylene for 24h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With molecular sevies 4A; tetrachlorobis(tetrahydrofuran)hafnium(IV) In o-xylene for 24h; Heating / reflux; | 97% |
| With molecular sevies 4A; tetrachlorobis(tetrahydrofuran)hafnium(IV) In o-xylene for 24h; Heating / reflux; | 96% |
-
-
123-99-9
azelaic acid

-
-
6080-56-4
lead(II) acetate trihydrate

-
-
7717-58-0, 14489-30-6, 25725-29-5, 87720-72-7
lead(II) azelate

| Conditions | Yield |
|---|---|
| In water High Pressure; placed in a bomb, heated to 220°C (3 h), held for 3 h; cooled to room temp. (3 h), crysts., washed (water), air dried; elem. anal.; | 96% |

| Conditions | Yield |
|---|---|
| With hafnium(IV) trifluoromethanesulfonate In toluene at 110℃; for 48h; Inert atmosphere; | 96% |

| Conditions | Yield |
|---|---|
| With DOWEX 50W-X2 In Petroleum ether for 4.5h; Heating; | 95% |

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate In propan-1-ol at 40℃; for 1.5h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: L-arginine; germanium dioxide In water at 85 - 95℃; for 1h; Stage #2: azelaic acid In water for 2h; | 95% |


| Conditions | Yield |
|---|---|
| With dmap; N-(3-dimethylaminopropyl)-N-ethylcarbodiimide In dichloromethane | 95% |
-
-
123-99-9
azelaic acid

-
-
91-16-7
1,2-dimethoxybenzene

-
-
32246-69-8
1,7-bis(3,4-dimethoxyphenyl)heptane-1,7-dione

| Conditions | Yield |
|---|---|
| Stage #1: azelaic acid With thionyl chloride In dichloromethane for 4.5h; Reflux; Stage #2: 1,2-dimethoxybenzene With aluminum (III) chloride In dichloromethane at 0℃; for 5h; Friedel-Crafts Acylation; | 95% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In 1-methyl-pyrrolidin-2-one at 170 - 230℃; for 18h; Temperature; | 95% |

| Conditions | Yield |
|---|---|
| In methanol at 21℃; for 48h; | 94% |

| Conditions | Yield |
|---|---|
| With sulfuric acid at 30 - 40℃; for 0.5h; | 93% |

| Conditions | Yield |
|---|---|
| With sulfuric acid | 92% |
Azelaic acid Chemical Properties
The Molecular Structure of Azelaic acid (CAS NO.123-99-9):
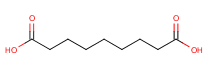
Empirical Formula: C9H16O4
Molecular Weight: 188.2209
IUPAC Name: nonanedioic acid
Appearance: white to cream solid
Nominal Mass: 188 Da
Average Mass: 188.2209 Da
Monoisotopic Mass: 188.104859 Da
Index of Refraction: 1.475
Molar Refractivity: 46.87 cm3
Molar Volume: 166.3 cm3
Surface Tension: 46.2 dyne/cm
Density: 1.131 g/cm3
Flash Point: 192.1 °C
Enthalpy of Vaporization: 67.8 kJ/mol
Boiling Point: 370.5 °C at 760 mmHg
Vapour Pressure: 1.66E-06 mmHg at 25°C
Water Solubility: 2.4 g/L (20 °C)
Stability: Stable. Combustible. Incompatible with bases, strong oxidizing agents. Readily biodegrades in soil and water with >70% DOC reduction after 28 days
Product Categories: API intermediates;alpha,omega-Alkanedicarboxylic Acids;alpha,omega-Bifunctional Alkanes;Monofunctional & alpha,omega-Bifunctional Alkanes
InChI
InChI=1/C9H16O4/c10-8(11)6-4-2-1-3-5-7-9(12)13/h1-7H2,(H,10,11)(H,12,13)
Smiles
C(CCCCCCCC(O)=O)(O)=O
Azelaic acid Uses
Azelaic acid (CAS NO.123-99-9) can be used as plasticizers, and used in the synthesis of alkyd resin, paint and other chemicals.
Azelaic acid Toxicity Data With Reference
| 1. | skn-rbt 500 mg/24 H MLD | EMERY* Emery Industries, Inc., Data Sheets. (4900 Este Ave., Cincinnati, OH 45232) S3B,- ,1964. | ||
| 2. | eye-rbt 3 mg MLD | EMERY* Emery Industries, Inc., Data Sheets. (4900 Este Ave., Cincinnati, OH 45232) S3B,- ,1964. | ||
| 3. | orl-rat LD50:>5 g/kg | NTIS** National Technical Information Service. (Springfield, VA 22161) (Formerly U.S. Clearinghouse for Scientific and Technical Information) AD-A067-313 . |
Azelaic acid Consensus Reports
Reported in EPA TSCA Inventory.
Azelaic acid Safety Profile
Hazard Codes:  Xi
Xi
Risk Statements: 36/37/38
R36/37/38: Irritating to eyes, respiratory system and skin
Safety Statements: 24/25-36-26
S24/25: Avoid contact with skin and eyes
S36: Wear suitable protective clothing
S26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
WGK Germany: 1
RTECS: CM1980000
HS Code: 29171390
Low toxicity by ingestion. A skin and eye irritant. Closely related to glutaric acid and adipic acid. Combustible when exposed to heat or flame; can react with oxidizing materials.
Azelaic acid Analytical Methods
For occupational chemical analysis use NIOSH: Azelaic Acid , 5019.
Azelaic acid Specification
Azelaic acid (CAS NO.123-99-9) is also called as Azelaic acid [USAN:INN] ; 1,7-Heptanedicarboxylic acid ; 1,9-Nonanedioic acid ; Acide azelaique ; Acide azelaique [French] ; Acidum azelaicum ; Azelaic acid, technical grade ; Heptanedicarboxylic acid ; Nonanedioic acid .
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View