-
Name
Azelastine
- EINECS 253-720-4
- CAS No. 58581-89-8
- Article Data7
- CAS DataBase
- Density 1.25 g/cm3
- Solubility
- Melting Point
- Formula C22H24ClN3O
- Boiling Point 533.9 °C at 760 mmHg
- Molecular Weight 381.905
- Flash Point 276.7 °C
- Transport Information
- Appearance white crystal powder
- Safety
- Risk Codes R50/53; R23/25
-
Molecular Structure
-
Hazard Symbols
 N,
N, T
T
- Synonyms 4-[(4-chlorophenyl)methyl]-2-(1-methylazepan-4-yl)-phthalazin-1-one;
- PSA 38.13000
- LogP 4.23540
Synthetic route
-
-
53242-88-9
4-[(4-chlorophenyl)methyl]-1(2H)-phthalazinone

-
B
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| With sodium hydroxide at 70℃; for 1h; Yield given; |

-
-
53242-76-5
2‐(4-chlorophenylacetyl)benzoic acid

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| With hydrogenchloride; potassium hydroxide 1.) reflux, 3 h, 2.) methanol, reflux, 2 h; Yield given. Multistep reaction; |
-
-
1878-66-6
4-chlorophenylacetic Acid

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: CH3COONa / 3 h / 245 - 250 °C 2: aq. NaOH / 2 h / Heating 3: 1.) 23percent aq. HCl, 2.) KOH / 1.) reflux, 3 h, 2.) methanol, reflux, 2 h View Scheme | |
| Multi-step reaction with 4 steps 1: CH3COONa / 3 h / 245 - 250 °C 2: aq. NaOH / 2 h / Heating 3: 90 percent / hydrazine sulfate, aq. NaOH / 2 h / Heating 4: aq. NaOH / 1 h / 70 °C View Scheme |
-
-
1859-33-2
1-methyl-1,2,3,5,6,7-hexahydroazepin-4-one

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 2.) NaBH4 / 1.) CH3OH, room temp. 2: 1.) 23percent aq. HCl, 2.) KOH / 1.) reflux, 3 h, 2.) methanol, reflux, 2 h View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: acetonitrile / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme |
-
-
85-44-9
phthalic anhydride

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: CH3COONa / 3 h / 245 - 250 °C 2: aq. NaOH / 2 h / Heating 3: 1.) 23percent aq. HCl, 2.) KOH / 1.) reflux, 3 h, 2.) methanol, reflux, 2 h View Scheme | |
| Multi-step reaction with 4 steps 1: CH3COONa / 3 h / 245 - 250 °C 2: aq. NaOH / 2 h / Heating 3: 90 percent / hydrazine sulfate, aq. NaOH / 2 h / Heating 4: aq. NaOH / 1 h / 70 °C View Scheme |
-
-
105279-16-1
(Z)-3-(4-chlorophenyl)methylidenephthalide

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: aq. NaOH / 2 h / Heating 2: 1.) 23percent aq. HCl, 2.) KOH / 1.) reflux, 3 h, 2.) methanol, reflux, 2 h View Scheme | |
| Multi-step reaction with 3 steps 1: aq. NaOH / 2 h / Heating 2: 90 percent / hydrazine sulfate, aq. NaOH / 2 h / Heating 3: aq. NaOH / 1 h / 70 °C View Scheme |
-
-
53242-76-5
2‐(4-chlorophenylacetyl)benzoic acid

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 90 percent / hydrazine sulfate, aq. NaOH / 2 h / Heating 2: aq. NaOH / 1 h / 70 °C View Scheme |

-
-
53242-76-5
2‐(4-chlorophenylacetyl)benzoic acid

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Stage #1: 1H-azepine, 4-hydrazinohexahydro-1-methyl, dihydrochloride; 2‐(4-chlorophenylacetyl)benzoic acid With sodium hydroxide In methanol; water for 4h; Heating / reflux; Stage #2: With hydrogenchloride In methanol; 2,8-dimethylnonan-5-one; water |
-
-
79307-93-0
azelastine hydrochloride

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water pH=10; Inert atmosphere; |

| Conditions | Yield |
|---|---|
| Stage #1: C14H23N3O(2+)*C4H2O4(2-) With hydrogenchloride at 20℃; for 2h; Reflux; Stage #2: 2‐(4-chlorophenylacetyl)benzoic acid for 2h; Reflux; | 8.1 g |

| Conditions | Yield |
|---|---|
| Stage #1: C14H23N3O(2+)*C4H2O4(2-) With hydrogenchloride at 20℃; for 2h; Reflux; Stage #2: 2‐(4-chlorophenylacetyl)benzoic acid for 2h; Reflux; | 7.5 g |

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 2.1: hydrogenchloride / 2 h / 20 °C / Reflux 2.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 2.1: hydrogenchloride / 2 h / 20 °C / Reflux 2.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 2 steps 1.1: acetonitrile / 1 h / 0 - 10 °C / Reflux 2.1: hydrogenchloride / 2 h / 20 °C / Reflux 2.2: 2 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: C14H23N3O(2+)*C4H4O4(2-) With hydrogenchloride at 20℃; for 2h; Reflux; Stage #2: 2‐(4-chlorophenylacetyl)benzoic acid for 2h; Reflux; | 8 g |
-
-
613-94-5
benzoic acid hydrazide

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: tetrahydrofuran / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme | |
| Multi-step reaction with 3 steps 1.1: potassium hydroxide / water / 1 h / 25 - 35 °C 1.2: 2 h / 0 - 10 °C 2.1: acetonitrile / 1 h / 0 - 10 °C / Reflux 3.1: hydrogenchloride / 2 h / 20 °C / Reflux 3.2: 2 h / Reflux View Scheme |

| Conditions | Yield |
|---|---|
| With C17H24N5Ru(1+)*F6P(1-); potassium acetate; potassium carbonate In 1-methyl-pyrrolidin-2-one at 35℃; for 72h; Inert atmosphere; | 91% |
-
-
58581-89-8
azelastine

-
-
79307-93-0
azelastine hydrochloride

| Conditions | Yield |
|---|---|
| With hydrogenchloride In toluene Reflux; | 87% |
-
-
10365-98-7
3-methoxyphenylboronic acid

-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| With 1,1'-bis-(diphenylphosphino)ferrocene; nickel(II) chloride hexahydrate; 1,8-diazabicyclo[5.4.0]undec-7-ene In methanol; N,N-dimethyl acetamide at 80℃; for 21h; Reagent/catalyst; Solvent; Inert atmosphere; Glovebox; | 51% |
-
-
100-58-3
phenylmagnesium bromide

-
-
58581-89-8
azelastine

-
-
103541-65-7
2-(1-benzylazepan-4-yl)-4-(4-chlorobenzyl)phthalazin-1(2H)-one

| Conditions | Yield |
|---|---|
| Stage #1: azelastine With 1,4-diaza-bicyclo[2.2.2]octane; tri(4-tolyl)aminium hexafluorophosphate In acetonitrile at -5℃; Inert atmosphere; Stage #2: phenylmagnesium bromide In tetrahydrofuran; acetonitrile at 0℃; Inert atmosphere; regioselective reaction; | 25% |

| Conditions | Yield |
|---|---|
| With pentamethylcyclopentadienyl(benzene)cobalt(III) hexafluorophosphate; potassium carbonate; silver carbonate In 2-methyltetrahydrofuran at 100℃; for 16h; Inert atmosphere; Sealed tube; | 25% |
-
-
58581-89-8
azelastine

| Conditions | Yield |
|---|---|
| With water In ethanol Heating / reflux; |

| Conditions | Yield |
|---|---|
| With mixed selector chiral stationary phase prepared with co-immobilized human serum albumin and cellulase on a poly(glycidylmethacrylate-co-ethylene glycol dimethacrylate) monolith In aq. phosphate buffer; isopropyl alcohol at 20℃; pH=7; Resolution of racemate; |
Azelastine Chemical Properties
Chemical Name: Azelastine
IUPAC Name: 4-[(4-Chlorophenyl)methyl]-2-(1-methylazepan-4-yl)phthalazin-1-one
CAS No.: 58581-89-8
Molecular Formula: C22H24ClN3O
Molecular Weight: 381.9 g/mol
Polar Surface Area: 35.91 Å2
Index of Refraction: 1.641
Molar Refractivity: 109.97 cm3
Molar Volume: 304.5 cm3
Surface Tension: 47.4 dyne/cm
Density: 1.25 g/cm3
Flash Point: 276.7 °C
Enthalpy of Vaporization: 81 kJ/mol
Boiling Point: 533.9 °C at 760 mmHg
Vapour Pressure: 1.77E-11 mmHg at 25°C
Following is the structure of Azelastine (CAS NO.58581-89-8):
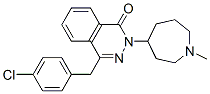
The chemical synonymous of Azelastine (CAS NO.58581-89-8) are 1(2h)-phthalazinone,4-((4-chlorophenyl)methyl)-2-(hexahydro-1-methyl-1h-azepin ; 4-(p-chlorobenzyl)-2-(hexahydro-1-methyl-1h-azepin-4-yl)-1-(2h)-phthalazinon ; AZELASTINE ; 1(2H)-Phthalazinone, 4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-yl)- ; 4-[(4-chlorophenyl)methyl]-2-(1-methylazepan-4-yl)-phthalazin-1-one ; CAS 790307-93-0 ; 4-[(4-chlorophenyl)methyl]-2-(hexahydro-1-methyl-1H-azepin-4-y1)-1(2H)-phthalazinone .
Azelastine Uses
Azelastine (CAS NO.58581-89-8) is used for bronchial asthma, nasalallergy treatment.and used as a nasal spray (Astelin or Astepro)for hay fever and as eye drops (Optivar) for allergicconjunctivitis.
Azelastine Toxicity Data With Reference
| dog | LD50 | intravenous | 13700ug/kg (13.7mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) GASTROINTESTINAL: NAUSEA OR VOMITING | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| dog | LD50 | oral | 51300ug/kg (51.3mg/kg) | BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD BEHAVIORAL: CHANGES IN MOTOR ACTIVITY (SPECIFIC ASSAY) GASTROINTESTINAL: NAUSEA OR VOMITING | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| mouse | LD50 | intramuscular | 59700ug/kg (59.7mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 1106, 1986. | |
| mouse | LD50 | intraperitoneal | 42800ug/kg (42.8mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| mouse | LD50 | intravenous | 25400ug/kg (25.4mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 1106, 1986. | |
| mouse | LD50 | oral | 124mg/kg (124mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| mouse | LD50 | subcutaneous | 54200ug/kg (54.2mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| rat | LD50 | intramuscular | 115mg/kg (115mg/kg) | Iyakuhin Kenkyu. Study of Medical Supplies. Vol. 17, Pg. 1106, 1986. | |
| rat | LD50 | intraperitoneal | 43200ug/kg (43.2mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| rat | LD50 | intravenous | 26900ug/kg (26.9mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| rat | LD50 | oral | 310mg/kg (310mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
| rat | LD50 | subcutaneous | 59600ug/kg (59.6mg/kg) | PERIPHERAL NERVE AND SENSATION: FLACCID PARALYSIS WITHOUT ANESTHESIA (USUALLY NEUROMUSCULAR BLOCKAGE) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Arzneimittel-Forschung. Drug Research. Vol. 31, Pg. 1184, 1981. |
Azelastine Safety Profile
Poison by ingestion, subcutaneous, intravenous, and intraperitoneal routes. Experimental reproductive effects. When heated to decomposition it emits toxic fumes of NOx and Cl−.
Azelastine Specification
Side effects of Azelastine (CAS NO.58581-89-8) spray vary somewhat depending on the condition being treated. The most common side effects include bitter taste, headache, rhinitis, nose bleed, and somnolence. Adults being treated for vasomotor rhinitis may also experience dysesthesia and sinusitis.
Related Products
- Azelastine
- Azelastine hydrochloride
- 585-81-9
- 58-58-2
- 5858-33-3
- 5858-39-9
- 585-84-2
- 58584-61-5
- 58584-94-4
- 5858-51-5
- 5858-53-7
- 58585-53-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View