-
Name
Azulene
- EINECS 205-993-6
- CAS No. 275-51-4
- Article Data81
- CAS DataBase
- Density 1.037 g/cm3
- Solubility Soluble in common solvents, insoluble in water
- Melting Point 98-100 °C(lit.)
- Formula C10H8
- Boiling Point 220.718 °C at 760 mmHg
- Molecular Weight 128.174
- Flash Point 76.66 °C
- Transport Information
- Appearance Blue Crystal
- Safety 61
- Risk Codes 51/53
-
Molecular Structure
-
Hazard Symbols
 N
N
- Synonyms Bicyclo[5.3.0]decapentaene;Cyclopentacycloheptene;NSC 89248;
- PSA 0.00000
- LogP 2.79140
Synthetic route
-
-
92622-71-4
tetracyclo<5.3.0.02.4.03.5>deca-6,8,10-triene

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In cyclohexane Quantum yield; Irradiation; | 100% |
| In (2)H8-toluene at 90 - 120℃; Thermodynamic data; Rate constant; ΔH (excit.), ΔS (excit.), Ea; |
-
-
607393-61-3
2-azulenyl trifluoromethanesulfonate

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With formic acid; tributyl-amine; tris-(o-tolyl)phosphine; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane for 24h; Heating; | 96% |

| Conditions | Yield |
|---|---|
| In 1,2-dichloro-ethane for 44h; Product distribution; Decomposition; retro-Diels-Alder reaction; Heating; | A 94% B 31% |

-
-
23306-02-7
1-chloroazulene

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With potassium phosphate; poly(methylhydrosiloxane); palladium diacetate In tetrahydrofuran at 80℃; for 12h; | 92% |

| Conditions | Yield |
|---|---|
| With trichloroacetic acid In benzene for 6h; Heating; | 90% |

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; cesium fluoride In 1,4-dioxane for 2h; Heating; | A 83% B 5% |


| Conditions | Yield |
|---|---|
| Stage #1: 6-bromoazulene With bis(tri-n-butyltin); tetrakis(triphenylphosphine) palladium(0) In toluene for 24h; Heating; Stage #2: para-nitrophenyl bromide With tri-tert-butyl phosphine; cesium fluoride; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane for 2h; Stille cross-coupling; Heating; Further stages.; | A 83% B 5% |

-
-
111-26-2
hexan-1-amine

-
-
620634-44-8
2-(1-azulenyl)-4,4,5,5-tetramethyl-[1,3,2]-dioxaborolane

-
-
563-96-2
2,2-dihydroxyacetic acid

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In methanol at 20℃; for 3h; Petasis Reaction; Inert atmosphere; | A 82% B 6% |

-
-
52866-26-9
((E)-Buta-1,3-dienyl)-diethyl-amine

-
-
82215-26-7
6-<(p-Nitrobenzoyl)oxy>fulvene

-
A
-
82215-27-8
6-(1-azulenyl)fulvene

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In benzene for 7h; Ambient temperature; | A n/a B 68% |


| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; cesium fluoride In 1,4-dioxane for 6h; Heating; | A 63% B 6% |


| Conditions | Yield |
|---|---|
| Stage #1: 6-bromoazulene With bis(tri-n-butyltin); tetrakis(triphenylphosphine) palladium(0) In toluene for 24h; Heating; Stage #2: 1-bromo-4-methoxy-benzene With tri-tert-butyl phosphine; cesium fluoride; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane for 6h; Stille cross-coupling; Heating; Further stages.; | A 63% B 6% |


| Conditions | Yield |
|---|---|
| at 600℃; under 0.000750075 - 0.0750075 Torr; for 0.833333h; | A 9% B 5% C 62% D 8% |


| Conditions | Yield |
|---|---|
| With diethylamine for 3h; Heating; | 60% |
-
-
92622-72-5
tricyclo<5.3.0.02,5>deca-3,6,8,10-tetraene

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In cyclohexane Quantum yield; Irradiation; | 60% |
| In (2)H8-toluene at 120 - 140℃; Thermodynamic data; Ea, half-life; |

| Conditions | Yield |
|---|---|
| With trifuran-2-yl-phosphane; tetraethylammonium iodide; zinc; bis(triphenylphosphine)nickel(II) chloride In tetrahydrofuran at 20℃; for 24h; | A 60% B n/a |
-
-
106-38-7
para-bromotoluene

-
-
35046-05-0
6-bromoazulene

-
A
-
73393-11-0
6,6'-Biazulenyl

-
B
-
102435-38-1
6-(p-Methylphenyl)azulen

-
C
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| Stage #1: 6-bromoazulene With bis(tri-n-butyltin); tetrakis(triphenylphosphine) palladium(0) In toluene for 24h; Heating; Stage #2: para-bromotoluene With tri-tert-butyl phosphine; cesium fluoride; tris(dibenzylideneacetone)dipalladium (0) In 1,4-dioxane for 24h; Stille cross-coupling; Heating; Further stages.; | A 12% B 60% C 5% |

-
-
106-38-7
para-bromotoluene

-
-
441292-61-1
6-(tri-n-butylstannyl)azulene

-
A
-
73393-11-0
6,6'-Biazulenyl

-
B
-
102435-38-1
6-(p-Methylphenyl)azulen

-
C
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; cesium fluoride In 1,4-dioxane for 24h; Heating; | A 31% B 58% C 10% |
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine In 1,4-dioxane for 24h; Heating; | A 32% B 27% C 18% |

-
-
14658-95-8
1,3-dibromoazulene

-
-
139-02-6
sodium phenoxide

-
A
-
78049-27-1
1-phenoxyazulene

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With copper(l) iodide In N,N-dimethyl-formamide at 150℃; for 6h; | A 15% B 55% |

-
-
61676-62-8
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
691897-66-2
6-iodoazulene

-
A
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| Stage #1: 6-iodoazulene With tri-n-butyllithium magnesate complex In diethyl ether for 0.5h; Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane In diethyl ether Further stages.; | A 8% B 55% |


| Conditions | Yield |
|---|---|
| With copper at 180℃; for 6h; | A 54% B n/a |
| With trifuran-2-yl-phosphane; tetraethylammonium iodide; zinc; bis(triphenylphosphine)nickel(II) chloride In tetrahydrofuran at 20℃; for 4h; | A 42% B 28% |
-
-
36044-42-5
1,3-diiodoazulene

-
-
122-39-4
diphenylamine

-
A
-
78049-26-0
1-diphenylaminoazulene

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With copper(l) iodide; copper; potassium carbonate at 160 - 170℃; for 2h; | A 19% B 54% |


| Conditions | Yield |
|---|---|
| Stage #1: 1,6-di-tert-butyl-3-iodoazulene With tri-n-butyllithium magnesate complex In diethyl ether for 0.5h; Stage #2: chloro-diphenylphosphine In diethyl ether Further stages.; | A 7% B 52% |

-
-
624-31-7
4-tolyl iodide

-
-
457644-50-7
2-(azulen-2-yl)-4,4,5,5- tetramethyl-1,3,2-dioxaborolane

-
A
-
111679-38-0
2-(4-tolyl)azulene

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; caesium carbonate In 1,4-dioxane at 80℃; for 24h; Miyaura-Suzuki cross-coupling; | A 51% B 4% |

-
-
61676-62-8
2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane

-
-
76279-71-5
1-azulenyl iodide

-
A
-
620634-44-8
2-(1-azulenyl)-4,4,5,5-tetramethyl-[1,3,2]-dioxaborolane

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| Stage #1: 1-azulenyl iodide With phenyllithium In tetrahydrofuran; diethyl ether; cyclohexane at -78℃; for 0.5h; Inert atmosphere; Stage #2: 2-Isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane In tetrahydrofuran; diethyl ether; cyclohexane at -78 - 20℃; for 3h; Inert atmosphere; | A 51% B n/a |

-
-
52487-41-9
1,2,3,4-tetrahydroazulen-1-one

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With methanesulfonic acid; phosphorus pentoxide at 60℃; | 50% |
| Multi-step reaction with 2 steps 1: 87 percent / aluminum hydride / tetrahydrofuran / 0.5 h / 0 °C 2: 37 percent / 10percent Pd/C / benzene; hexane / 480 °C View Scheme |

| Conditions | Yield |
|---|---|
| In acetic acid for 2h; from boiling point to r.t.; | A 24 mg B 50% |


| Conditions | Yield |
|---|---|
| With pyrrole In acetic acid at 20℃; for 72h; decarbonylation; | 49% |
-
-
106-37-6
1.4-dibromobenzene

-
-
457644-50-7
2-(azulen-2-yl)-4,4,5,5- tetramethyl-1,3,2-dioxaborolane

-
A
-
19227-07-7
2-phenylazulene

-
B
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine; caesium carbonate In 1,4-dioxane at 80℃; for 24h; Miyaura-Suzuki cross-coupling; | A 2% B 2% C 44% |


| Conditions | Yield |
|---|---|
| at 500℃; under 0.001 Torr; Product distribution; | A n/a B 42% |
-
-
106-43-4
para-chlorotoluene

-
-
457644-50-7
2-(azulen-2-yl)-4,4,5,5- tetramethyl-1,3,2-dioxaborolane

-
A
-
82893-99-0
2,2′-biazulene

-
B
-
111679-38-0
2-(4-tolyl)azulene

-
C
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With barium dihydroxide; cesium carbonate; tris(dibenzylideneacetone)dipalladium (0); tri-tert-butyl phosphine In 1,4-dioxane at 80℃; for 24h; | A 40% B 12% C 4% |

-
-
275-51-4
azulene

-
-
14658-95-8
1,3-dibromoazulene

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; acetic anhydride In diethyl ether | 100% |
| With N-Bromosuccinimide In benzene for 2h; Ambient temperature; | 95% |
| With N-Bromosuccinimide In hexane at 20℃; | 89% |
-
-
275-51-4
azulene

-
-
76279-71-5
1-azulenyl iodide

| Conditions | Yield |
|---|---|
| With N-iodo-succinimide In dichloromethane at 0℃; for 0.25h; Inert atmosphere; | 100% |
| With N-iodo-succinimide In dichloromethane at 20℃; for 2h; | 98% |
| With N-iodo-succinimide In dichloromethane at 0℃; for 0.25h; Inert atmosphere; | 87% |
-
-
356-42-3
2,2,3,3,3-pentafluoropropanoic anhydride

-
-
275-51-4
azulene

-
-
73017-88-6
1-(pentafluoropropionyl)azulene

| Conditions | Yield |
|---|---|
| for 0.25h; | 100% |
-
-
275-51-4
azulene

-
-
36044-42-5
1,3-diiodoazulene

| Conditions | Yield |
|---|---|
| With aluminum oxide; iodine In solid for 20h; Ambient temperature; | 100% |
| With N-iodo-succinimide In dichloromethane-d2 | 98% |
| With N-iodo-succinimide In benzene at 50℃; for 3h; | 96% |

| Conditions | Yield |
|---|---|
| With Pd/C; hydrogen In chloroform at 20℃; under 760.051 Torr; for 5h; Catalytic behavior; | 100% |
| With lithium; nickel dichloride In tetrahydrofuran at 20℃; Reduction; | 72 % Chromat. |
-
-
260-94-6
acridine

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
275-51-4
azulene

-
-
929021-11-4
C38H24F6N2O4S2

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 99% |
| In dichloromethane at 20℃; for 2h; | 99% |

-
-
1193-82-4
racemic methyl phenyl sulfoxide

-
-
407-25-0
trifluoroacetic anhydride

-
-
275-51-4
azulene

-
-
1029874-10-9
(1-azulenyl)methylphenylsulfonium trifluoroacetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 0.166667h; | 99% |

-
-
945-51-7
1,1'-sulfinylbisbenzene

-
-
407-25-0
trifluoroacetic anhydride

-
-
275-51-4
azulene

-
-
1029874-04-1
(1-azulenyl)diphenylsulfonium trifluoroacetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 99% |

-
-
67-68-5
dimethyl sulfoxide

-
-
407-25-0
trifluoroacetic anhydride

-
-
275-51-4
azulene

-
-
1029874-01-8
(1-azulenyl)dimethylsulfonium trifluoroacetate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 1h; | 99% |


| Conditions | Yield |
|---|---|
| Stage #1: dimethyl sulfoxide; azulene With trifluoromethylsulfonic anhydride In dichloromethane at 20℃; for 0.5h; Stage #2: With triethylamine In ethanol for 2h; Reflux; | 98% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 97% |
| In dichloromethane at 20℃; for 2h; | 97% |

-
-
95-16-9
1,3-Benzothiazole

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
275-51-4
azulene

-
-
929021-13-6
C26H16F6N2O4S4

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 97% |

-
-
1203707-11-2
1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}-2-(3-guaiazulenyl)ethylene

-
-
275-51-4
azulene

-
A
-
489-84-9
7-isopropyl-1,4-dimethyl-azulene

-
B
-
1314045-03-8
2-(azulen-1-yl)-1,1-bis{4-[2-(dimethylamino)ethoxy]phenyl}ethylene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water; acetonitrile at 60℃; for 3h; | A 97% B 58% |

-
-
769-42-6
1,3-dimethylbarbituric acid

-
-
101906-05-2
2,2-dihydroxy-1-[4-(trifluoromethyl)phenyl]ethanone

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In isopropyl alcohol at 80℃; | 97% |


| Conditions | Yield |
|---|---|
| With trichlorophosphate In N,N-dimethyl-formamide at 0 - 20℃; | 95% |
| With Vilsmeier reagent In dichloromethane at 0 - 25℃; for 0.75h; Inert atmosphere; | 90% |
| With N,N-dimethyl-formamide; trichlorophosphate | |
| With N-methyl-N-phenylformamide | |
| Multi-step reaction with 2 steps 1: aq. HClO4 / ethanol 2: aq. KMnO4 / acetone View Scheme |

| Conditions | Yield |
|---|---|
| Stage #1: N,N-dimethyl-formamide With trichlorophosphate at 0 - 20℃; for 0.5h; Stage #2: azulene at 20℃; for 1h; | 95% |
| With trichlorophosphate at 0 - 20℃; for 1.5h; | 95% |
| Stage #1: N,N-dimethyl-formamide With phosphorus trichloride at 0℃; for 0.5h; Stage #2: azulene In N,N-dimethyl-formamide at 25℃; for 2h; | 95% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; | 95% |


| Conditions | Yield |
|---|---|
| Stage #1: 2,6-dimethylpyrone With trichlorophosphate In nitromethane for 1h; Stage #2: azulene In nitromethane for 0.25h; Stage #3: With perchloric acid In nitromethane at 70 - 80℃; for 0.333333h; | 94% |
-
-
66-71-7
1,10-Phenanthroline

-
-
358-23-6
trifluoromethylsulfonic anhydride

-
-
275-51-4
azulene

-
-
929021-12-5
C36H22F6N4O4S2

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | 94% |
| In dichloromethane at 20℃; for 2h; | 94% |


-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With dirhodium tetraacetate In 1,2-dichloro-ethane at 60℃; for 12h; diastereoselective reaction; | 94% |
| With rhodium (II) octanoate dimer In 1,2-dichloro-ethane at 60℃; for 3h; | 94% |
-
-
275-51-4
azulene

-
-
63320-32-1
1,6-dihydroazulene

| Conditions | Yield |
|---|---|
| With ammonia; sodium In diethyl ether at -78℃; for 0.5h; Birch reduction; | 93% |
-
-
275-51-4
azulene

-
-
741737-71-3
1,1-bis[4-(methoxy)phenyl]-2-(3-guaiazulenyl)ethylene

-
A
-
489-84-9
7-isopropyl-1,4-dimethyl-azulene

-
B
-
1314045-02-7
1,3-bis[2,2-bis(4-methoxyphenyl)ethenyl]azulene

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol; water; acetonitrile at 60℃; for 3h; | A 93% B 19% C 34% |

-
-
22979-35-7
Methyl phenyldiazoacetate

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| With copper(II) hexafluoroacetylacetonate In 1,2-dichloro-ethane at 20℃; for 0.5h; | 93% |
-
-
53535-47-0
2-diazo-1,3-dicyano-6-oxo-2,6-azulenequinone

-
-
275-51-4
azulene

| Conditions | Yield |
|---|---|
| In ethyl acetate for 1.5h; Condensation; dediazotization; Photolysis; | A 1% B 92% C 5% |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; | A 92% B 6% |
| In dichloromethane at 20℃; for 0.5h; | A 92% B 6% |


| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; | 92% |

Azulene History
Dating back to the 15th century as the azure-blue chromophore, Azulene (CAS NO.275-51-4) obtained by steam distillation of German chamomile.Its structure and first synthesis were reported by Lavoslav Ru?i?ka, followed in 1937 by Placidus Plattner.The chromophore was discovered in yarrow and wormwood and named in 1863 by Septimus Piesse.
Azulene Consensus Reports
Reported in EPA TSCA Inventory.
Azulene Specification
Azulene is an organic compound with the formula C10H8, and its systematic name is the same with the product name. With the CAS registry number 275-51-4, it is also named as Cyclopentacycloheptene. It belongs to the product categories of Aromatic Hydrocarbons (substituted) & Derivatives; Tropolones & Azulenes; Azulenes. Its EINECS number is 205-993-6. In addition, the molecular weight is 128.17. Its classification codes are: (1)Analgesics; (2)Analgesics, Non-Narcotic; (3)Anti-Inflammatory Agents; (4)Anti-inflammatory agents, non-steroidal; (5)Antirheumatic Agents; (6)Peripheral Nervous System Agents; (7)Sensory System Agents. This chemical should be sealed and stored in a cool and dry place. Moreover, it should be protected from light. In organometallic chemistry, azulene serves as a ligand for low-valent metal centers. It is reported to be anti-inflammatory principle of Matricaria. When heated, it can rearrange into naphthalene.
Physical properties of Azulene are: (1)ACD/LogP: 3.361; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 3.36; (4)ACD/LogD (pH 7.4): 3.36; (5)ACD/BCF (pH 5.5): 211.02; (6)ACD/BCF (pH 7.4): 211.02; (7)ACD/KOC (pH 5.5): 1604.58; (8)ACD/KOC (pH 7.4): 1604.58; (9)#H bond acceptors: 0; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 0; (12)Index of Refraction: 1.632; (13)Molar Refractivity: 44.095 cm3; (14)Molar Volume: 123.549 cm3; (15)Polarizability: 17.481×10-24cm3; (16)Surface Tension: 40.2 dyne/cm; (17)Density: 1.037 g/cm3; (18)Flash Point: 76.66 °C; (19)Enthalpy of Vaporization: 43.854 kJ/mol; (20)Boiling Point: 220.718 °C at 760 mmHg; (21)Vapour Pressure: 0.17 mmHg at 25°C.
Preparation: this chemical can be prepared by azulene-1-carboxylic acid by heating. This reaction will need reagent CCl3COOH and solvent benzene with the reaction time of 6 hours. The yield is about 90%.
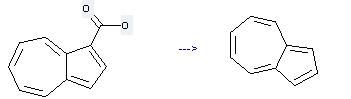
Uses of Azulene: it can be used to produce 1,3-dibromo-azulene at the ambient temperature. It will need reagent NBS and solvent benzene with the reaction time of 2 hours. The yield is about 95%.
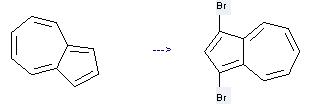
When you are using this chemical, please be cautious about it as the following:
This chemcial is toxic to aquatic organisms as it may cause long-term adverse effects in the aquatic environment. You must avoid releasing it to the environment, and you need to refer to special instructions/safety data sheet.
You can still convert the following datas into molecular structure:
(1)SMILES: c1cccc2cccc2c1
(2)Std. InChI: InChI=1S/C10H8/c1-2-5-9-7-4-8-10(9)6-3-1/h1-8H
(3)Std. InChIKey: CUFNKYGDVFVPHO-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 108mg/kg (108mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. | |
| mouse | LD50 | intravenous | 56mg/kg (56mg/kg) | U.S. Army Armament Research & Development Command, Chemical Systems Laboratory, NIOSH Exchange Chemicals. Vol. NX#07952, | |
| mouse | LD50 | oral | > 3gm/kg (3000mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. | |
| mouse | LD50 | subcutaneous | 145mg/kg (145mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. | |
| rat | LD50 | intraperitoneal | 180mg/kg (180mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. | |
| rat | LD50 | oral | > 4gm/kg (4000mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. | |
| rat | LD50 | subcutaneous | 520mg/kg (520mg/kg) | Drugs in Japan Vol. 6, Pg. 13, 1982. |
Related Products
- Azulene
- Azulene, 4-(iodomethyl)-
- Azulene, 4-methyl-
- 27552-97-2
- 27554-26-3
- 27555-23-3
- 27555-34-6
- 27563-65-1
- 2756-56-1
- 27568-04-3
- 27568-05-4
- 2756-85-6
- 2756-87-8
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View