-
Name
3,4-DIMETHOXYSTYRENE
- EINECS 228-962-9
- CAS No. 6380-23-0
- Article Data81
- CAS DataBase
- Density 1.004 g/cm3
- Solubility
- Melting Point
- Formula C10H12O2
- Boiling Point 259.4 °C at 760 mmHg
- Molecular Weight 164.204
- Flash Point 95.9 °C
- Transport Information
- Appearance
- Safety 26-36/37
- Risk Codes 36
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Styrene,3,4-dimethoxy- (7CI,8CI);Veratrole, 4-vinyl- (6CI);1,2-Dimethoxy-4-vinylbenzene;3,4-Dimethoxystyrene;3,4-Dimethoxystyrol;4-Vinyl-1,2-dimethoxybenzene;4-Vinylveratrole;
- PSA 18.46000
- LogP 2.34680
Synthetic route
-
-
61078-14-6
1-methyl-2-(methylsulfonyl)-1H-benzo[d]imidazole

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With sodium hexamethyldisilazane In N,N-dimethyl-formamide at -55 - 20℃; Reagent/catalyst; Temperature; Time; Inert atmosphere; | 97% |
| With sodium hexamethyldisilazane In tetrahydrofuran; N,N-dimethyl-formamide at -55 - 20℃; for 2h; Reagent/catalyst; Temperature; Inert atmosphere; | 97% |
-
-
21852-32-4
4-bromomethyl-1,2-dimethoxybenzene

-
-
2181-42-2
trimethylsulphonium iodide

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With n-butyllithium; lithium iodide In tetrahydrofuran; hexane from 0 degC to room temp.; | 95% |
-
-
21852-32-4
4-bromomethyl-1,2-dimethoxybenzene

-
-
47025-43-4, 19365-61-8
methylenetriphenylarsorane

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran; N,N,N,N,N,N-hexamethylphosphoric triamide; hexane for 1h; Ambient temperature; | 95% |
-
-
141-82-2
malonic acid

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
14737-89-4
3,4-dimethoxy-trans-cinnamic acid

| Conditions | Yield |
|---|---|
| With pyridine; acetic acid at 130℃; for 0.133333h; Knoevenagel-Doebner reaction; microwave irradiation; | A 4 % Spectr. B 91% |


| Conditions | Yield |
|---|---|
| With platinum on carbon; potassium tert-butylate; hydrogen In toluene under 1875.19 Torr; for 15h; Inert atmosphere; Reflux; | 91% |
| With C15H25Cl2N3NiO3; potassium tert-butylate In toluene at 110℃; for 12h; Catalytic behavior; Reagent/catalyst; Temperature; Schlenk technique; Inert atmosphere; | 76% |
-
-
1779-49-3
Methyltriphenylphosphonium bromide

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With n-butyllithium In tetrahydrofuran at -78℃; for 4h; Inert atmosphere; | 90% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane; cyclohexane at 0℃; for 4h; Inert atmosphere; Stage #2: 3,4-dimethoxy-benzaldehyde In tetrahydrofuran; hexane; cyclohexane at 20℃; for 10h; Wittig Olefination; Inert atmosphere; | 89% |
| Stage #1: Methyltriphenylphosphonium bromide With n-butyllithium In tetrahydrofuran; hexane at 0℃; for 2h; Schlenk technique; Stage #2: 3,4-dimethoxy-benzaldehyde In tetrahydrofuran at 0 - 20℃; | 89% |

| Conditions | Yield |
|---|---|
| With Hoveyda-Grubbs catalyst second generation; di-μ-bromobis-(tritert-butylphosphine)dipalladium(I) In tetrahydrofuran at 60℃; under 7500.75 Torr; for 16h; Autoclave; | 90% |
-
-
13170-43-9
(trimethylsilyl)methylmagnesium chloride

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: (trimethylsilyl)methylmagnesium chloride; 3,4-dimethoxy-benzaldehyde In tetrahydrofuran at 0 - 20℃; Peterson Olefination; Inert atmosphere; Stage #2: With bis(trifluoromethanesulfonyl)amide In 1,2-dichloro-ethane at 20℃; for 0.25h; Peterson Olefination; | 89% |
-
-
2065-66-9
methyl-triphenylphosphonium iodide

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| Stage #1: methyl-triphenylphosphonium iodide With potassium tert-butylate In tetrahydrofuran at 0℃; for 1h; Stage #2: 3,4-dimethoxy-benzaldehyde In tetrahydrofuran at 20℃; for 24h; | 87% |
| Stage #1: methyl-triphenylphosphonium iodide With potassium tert-butylate In tetrahydrofuran for 1h; Inert atmosphere; Stage #2: 3,4-dimethoxy-benzaldehyde In tetrahydrofuran at 25℃; for 24h; Wittig reaction; Inert atmosphere; | 75.2% |
| With tert-butoxide In tetrahydrofuran at 0℃; Wittig reaction; | 60% |
-
-
3112-85-4
Methyl phenyl sulfone

-
-
93-03-8
(3,4-dimethoxyphenyl)methanol

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With sodium hydride In mineral oil at 135℃; for 7h; Inert atmosphere; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| With sodium hydroxide In methanol; water at 100℃; for 8h; Hiyama Coupling; Sealed tube; | 78% |
-
-
124-38-9
carbon dioxide

-
-
120-20-7
2-(3,4-dimethoxyphenyl)-ethylamine

-
-
616-38-6
carbonic acid dimethyl ester

-
B
-
6380-23-0
3,4-dimethoxystyrene

-
C
-
3490-05-9
N,N-dimethyl-3,4-dimethoxy-β-phenethylamine

-
D
-
35690-71-2
[2-(3,4-dimethoxy-phenyl)-ethyl]-carbamic acid methyl ester

| Conditions | Yield |
|---|---|
| at 130℃; under 90007.2 - 97507.8 Torr; for 24h; | A n/a B n/a C n/a D 77% |


| Conditions | Yield |
|---|---|
| With 1-hexyl-3-methyl-1-imidazolium bromide at 140℃; for 0.2h; Microwave irradiation; Combinatorial reaction / High throughput screening (HTS); chemoselective reaction; | 74% |
| With toluene-4-sulfonic acid In chloroform for 8h; | 58% |
| Multi-step reaction with 2 steps 1: diethyl ether; CaCl2; hydrogen chloride / 0 °C 2: pyridine View Scheme | |
| at 120℃; for 0.25h; Microwave irradiation; Ionic liquid; |
-
-
67-71-0
dimethylsulfone

-
-
93-03-8
(3,4-dimethoxyphenyl)methanol

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; potassium tert-butylate; iron(II) chloride In toluene at 120℃; for 24h; Julia Olefin Synthesis; Inert atmosphere; Schlenk technique; | A 74% B n/a |

-
-
120-14-9
3,4-dimethoxy-benzaldehyde

-
-
19493-09-5
Methylenetriphenylphosphorane

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| In 1,4-dioxane; water Inert atmosphere; | 73% |
| Wittig reaction; | |
| at 20℃; for 10h; Wittig Olefination; |
-
-
67-71-0
dimethylsulfone

-
-
93-03-8
(3,4-dimethoxyphenyl)methanol

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
30405-75-5
4-isopropenyl-1,2-dimethoxybenzene

| Conditions | Yield |
|---|---|
| With C15H25Br2N3NiO3; potassium tert-butylate In toluene at 110℃; for 12h; Catalytic behavior; Reagent/catalyst; Solvent; Schlenk technique; Inert atmosphere; | A 72% B 6% |
| With C24H20ClN2OPRu; potassium tert-butylate In 1,4-dioxane at 125℃; for 5h; Inert atmosphere; Schlenk technique; Glovebox; | A 69% B 10 %Spectr. |


| Conditions | Yield |
|---|---|
| bis-triphenylphosphine-palladium(II) chloride In tetrahydrofuran at 60℃; for 12h; | 59% |
-
-
39792-99-9
[2-(3,4-dimethoxyphenyl)-ethyl](ethyl)amine

-
-
106-89-8
epichlorohydrin

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| In chlorobenzene at 115℃; | 56% |
-
-
40173-90-8
3,4-dimethoxyphenethyl bromide

-
-
299-42-3
ephedrine

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
130459-47-1
(-)-(1R,2S)-N-(3,4-dimethoxyphenethyl)ephedrine

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetonitrile for 48h; Heating; | A 54% B 34% |


| Conditions | Yield |
|---|---|
| In chlorobenzene at 115℃; | 47% |
-
-
40173-90-8
3,4-dimethoxyphenethyl bromide

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
1087319-37-6
2-[2-(3,4-dimethoxyphenyl)ethyl]-1-(1-phenylethyl)-1,2,3,4-tetrahydroquinoline-2-carbonitrile

| Conditions | Yield |
|---|---|
| Stage #1: 1-(1-phenylethyl)-1,2,3,4-tetrahydroquinoline-2-carbonitrile With lithium diisopropyl amide In tetrahydrofuran; hexane at -78 - -20℃; Inert atmosphere; Stage #2: 3,4-dimethoxyphenethyl bromide In tetrahydrofuran; Hexachlorobutadiene at -60 - 20℃; for 12h; | A n/a B 35% |

-
-
40173-90-8
3,4-dimethoxyphenethyl bromide

-
-
321-97-1
(1R,2R)-pseudoephedrine

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
130459-44-8
(-)-(1R,2R)-N-(3,4-dimethoxyphenethyl)pseudoephedrine

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; sodium iodide In ethanol for 60h; Heating; | A n/a B 29% |

-
-
109-97-7
pyrrole

-
-
40173-90-8
3,4-dimethoxyphenethyl bromide

-
B
-
6380-23-0
3,4-dimethoxystyrene

-
C
-
56014-51-8
1-[2-(3,4-dimethoxyphenyl)ethyl]-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: pyrrole With potassium hydroxide; 18-crown-6 ether In benzene for 2h; Heating; Stage #2: 3,4-dimethoxyphenethyl bromide In benzene Heating; Further stages.; | A 23% B 27% C 27% |

-
-
109-97-7
pyrrole

-
-
75010-39-8
1-(3,4-dimethoxyphenyl)-2-(p-toluenesulfonyloxy)ethane

-
B
-
6380-23-0
3,4-dimethoxystyrene

-
C
-
56014-51-8
1-[2-(3,4-dimethoxyphenyl)ethyl]-1H-pyrrole

| Conditions | Yield |
|---|---|
| Stage #1: pyrrole With potassium hydroxide; 18-crown-6 ether In benzene for 2h; Heating; Stage #2: 1-(3,4-dimethoxyphenyl)-2-(p-toluenesulfonyloxy)ethane In benzene Heating; Further stages.; | A 4% B 4% C 9% |

-
-
110-86-1
pyridine

-
-
50919-04-5
4-(1-chloro-ethyl)-1,2-dimethoxy-benzene

-
-
6380-23-0
3,4-dimethoxystyrene
-
-
50919-04-5
4-(1-chloro-ethyl)-1,2-dimethoxy-benzene

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With pyridine |

| Conditions | Yield |
|---|---|
| und Destillation des Reaktionsprodukts; | |
| With diethyl ether Erhitzen des nach der Hydrolyse erhaltenen Reaktionsprodukts unter vermindertem Druck auf Siedetemperatur; |
-
-
14737-89-4
3,4-dimethoxy-trans-cinnamic acid

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With quinoline; copper(II) sulfate at 220℃; |
-
-
1019628-47-7
N-<3,4-Dimethoxy-α-methyl-benzyl>-glycin-methyl-ester

-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol |
-
-
120-93-4
imidazolidone

-
-
40173-90-8
3,4-dimethoxyphenethyl bromide

-
A
-
6380-23-0
3,4-dimethoxystyrene

-
B
-
74996-66-0
1-<2-(3,4-dimethoxyphenyl)ethyl>imidazolidin-2-one

| Conditions | Yield |
|---|---|
| With sodium hydride 1) benzene, reflux, 4 h 2) benzene, reflux, 16 h; Yield given. Multistep reaction. Yields of byproduct given; |


| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; C21H19N5Pd(2+)*2BF4(1-) In decane; acetonitrile at 45℃; for 4h; Wacker Oxidation; | 100% |
| With perchloric acid; oxygen; palladium diacetate; p-benzoquinone; sodium nitrite In methanol; water at 20℃; for 24h; Wacker Oxidation; Schlenk technique; Sealed tube; Green chemistry; | 95% |
| With palladium diacetate; Dess-Martin periodane In water; acetonitrile at 50℃; Wacker-Tsuji Olefin Oxidation; Inert atmosphere; | 87% |
| With chromium(VI) oxide; palladium dichloride In water; acetonitrile at 20 - 60℃; for 24h; Wacker-Tsuji Olefin Oxidation; | 75% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; C22H24CuN4; dihydrogen peroxide In water at 40℃; for 7.5h; pH=2; Reagent/catalyst; |

| Conditions | Yield |
|---|---|
| With 4,4'-di-tert-butylbiphenyl; lithium; isopropyl alcohol; nickel dichloride In tetrahydrofuran at 20 - 76℃; Inert atmosphere; chemoselective reaction; | 99% |
| With hydrogen In methanol at 20℃; for 18h; chemoselective reaction; | 99% |
| With hydrogen at 80℃; under 7500.75 Torr; for 1h; Autoclave; | 94% |
| With hydrogen; platinum(IV) oxide In acetic acid | 54.9% |
| With palladium 10% on activated carbon; hydrogen at 20℃; for 18h; Sealed tube; | 83 mg |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
100-00-5
4-chlorobenzonitrile

-
-
51042-54-7
1,2-Dimethoxy-4-[2-(4-nitrophenyl)ethenyl]benzene

| Conditions | Yield |
|---|---|
| With C32H27N4O2Pd(1+)*BF4(1-); sodium acetate at 140℃; for 12h; Inert atmosphere; Schlenk technique; | 99% |
| With tetrabutylammomium bromide; palladium diacetate; silica gel; potassium carbonate for 0.75h; Neat (no solvent); | 87% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
326491-79-6
(R)-2-hydroxy-1-(3,4-dimethoxyphenyl)ethanol

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; potassium carbonate; potassium hexacyanoferrate(III); 1,4-bis(9-O-dihydroquinidine)phthalazine In water; toluene; tert-butyl alcohol at 0℃; for 24h; Sharpless asymmetric dihydroxylation; | 97% |
| With AD-mix β In water; tert-butyl alcohol at 0℃; Sharpless Dihydroxylation; |

| Conditions | Yield |
|---|---|
| With 2,6-di-tert-butyl-4-methyl-phenol at 80℃; for 120h; Diels-Alder cycloaddition; | 97% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
140687-75-8, 102104-61-0
2-(3,4-dimethoxyphenyl)oxirane

| Conditions | Yield |
|---|---|
| With 3,3-dimethyldioxirane In acetone for 0.666667h; Ambient temperature; | 96% |
| With PS-DVB supported phthalic anhydride; urea-hydrogen peroxide In dichloromethane at 20℃; | 77% |

| Conditions | Yield |
|---|---|
| With Cl2Ru=CHPh(PCy3)[bis(1,3-Mes-2-ylimidazolidine)] In dichloromethane at 40℃; for 12h; | 96% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
1192036-78-4
C20H24O6

| Conditions | Yield |
|---|---|
| With sodium hydrogencarbonate; 3-chloro-benzenecarboperoxoic acid In dichloromethane at 25℃; | 96% |

| Conditions | Yield |
|---|---|
| With formic acid; palladium diacetate; CyJohnPhos In toluene at 90℃; for 24h; Inert atmosphere; Sealed tube; regioselective reaction; | 96% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
90-14-2
1-Iodonaphthalene

-
-
23833-70-7
(E)-1-(3,4-dimethoxystyryl)naphthalene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; triethylamine; N,N-dimethyl-formamide; lithium chloride at 110℃; for 6h; Heck Reaction; Inert atmosphere; | 96% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
13443-56-6
β-hydroxy-3,4-dimethoxy phenethyl alcohol

| Conditions | Yield |
|---|---|
| With osmium(VIII) oxide; 4-methylmorpholine N-oxide In tetrahydrofuran; water; tert-butyl alcohol at 20℃; for 12h; Sealed tube; | 95% |
| With osmium(VIII) oxide; 4-methylmorpholine N-oxide In water; acetone at 20℃; for 16h; | 94% |
| With pyridine; osmium(VIII) oxide In diethyl ether at 20℃; for 20h; dihydroxylation; | 80% |
| Upjohn Dihydroxylation; Inert atmosphere; |
-
-
31617-39-7
1,3-diethyl-7-methyl-1H-purine-2,6(3H,7H)-dione

-
-
6380-23-0
3,4-dimethoxystyrene

-
-
155270-99-8
istradefylline

| Conditions | Yield |
|---|---|
| Stage #1: 1,3-diethyl-7-methyl-1H-purine-2,6(3H,7H)-dione With pyridine; palladium diacetate; copper(II) acetate monohydrate; copper(l) chloride In N,N-dimethyl acetamide at 20℃; for 0.0833333h; Heck reaction; Inert atmosphere; Stage #2: 3,4-dimethoxystyrene In N,N-dimethyl acetamide at 120℃; for 20h; Heck reaction; Inert atmosphere; | 95% |
-
-
104-92-7
1-bromo-4-methoxy-benzene

-
-
6380-23-0
3,4-dimethoxystyrene

-
-
263327-99-7
4,3’,4’-trimethoxystilbene

| Conditions | Yield |
|---|---|
| With C33H33N2(1+)*Cl(1-); palladium diacetate; potassium carbonate In water; N,N-dimethyl-formamide for 2h; Heck Reaction; Inert atmosphere; Sealed tube; Heating; | 95% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
41172-57-0
5-Methyloxazol-4-carbonsaeure-methylester

-
-
1096163-95-9
(E)-methyl 2-(3,4-dimethoxystyryl)-5-methyloxazole-4-carboxylate

| Conditions | Yield |
|---|---|
| With 1,10-Phenanthroline; palladium(II) trifluoroacetate; silver trifluoroacetate In toluene at 130℃; for 16h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: potassium cyanide With acetic acid In ethylene glycol at 60℃; Sealed tube; Stage #2: 3,4-dimethoxystyrene With bis(1,5-cyclooctadiene)nickel (0); 4,5-bis(diphenylphos4,5-bis(diphenylphosphino)-9,9-dimethylxanthenephino)-9,9-dimethylxanthene at 60℃; for 18h; Sealed tube; | 95% |
-
-
34418-91-2
2,3,4,5-tetrahydropyridine N-oxide

-
-
6380-23-0
3,4-dimethoxystyrene

-
-
79235-48-6
2-(3,4-Dimethoxy-phenyl)-hexahydro-isoxazolo[2,3-a]pyridine

| Conditions | Yield |
|---|---|
| In toluene Heating; | 93% |

| Conditions | Yield |
|---|---|
| With potassium chloride; tetrabutylammomium bromide; potassium carbonate; palladium dichloride In 1-methyl-pyrrolidin-2-one at 100℃; for 24h; Heck Reaction; Inert atmosphere; regioselective reaction; | 93% |

| Conditions | Yield |
|---|---|
| With 1,1'-bis(dicyclohexylphosphinocyclopentadienyl)iron; palladium diacetate; acetic acid In toluene at 90℃; for 24h; Inert atmosphere; Sealed tube; regioselective reaction; | 93% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
89845-28-3
2-(2-phenylethynyl)-1,4-benzoquinone

-
-
1432504-67-0
2-(3,4-dimethoxyphenyl)-7-(phenylethynyl)-2,3-dihydrobenzofuran-5-ol

| Conditions | Yield |
|---|---|
| With bismuth(lll) trifluoromethanesulfonate In acetonitrile at 0℃; Inert atmosphere; Schlenk technique; | 92% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
51698-53-4
2‐(3,4‐dimethoxyphenyl)propionitrile

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel (0); hydrogen cyanide; (3aR,8aR)-4,4,8,8-tetrakis(3,5-dimethylphenyl)-6-(2-(diphenylphosphino)-6-isopropylphenoxy)-2,2-dimethyltetrahydro-[1,3]dioxolo[4,5-e][1,3,2]dioxaphosphepine In tetrahydrofuran at 20℃; for 2h; Reagent/catalyst; Solvent; Inert atmosphere; Schlenk technique; Optical yield = 88 %ee; enantioselective reaction; | 92% |
-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate In acetonitrile at 20℃; for 0.166667h; regioselective reaction; | 92% |
-
-
6380-23-0
3,4-dimethoxystyrene

| Conditions | Yield |
|---|---|
| With bis-triphenylphosphine-palladium(II) chloride; triethylamine; N,N-dimethyl-formamide; lithium chloride at 110℃; for 5h; Heck Reaction; Inert atmosphere; | 92% |

| Conditions | Yield |
|---|---|
| With bis(1,5-cyclooctadiene)nickel(0); C33H30N2O2; lithium tert-butoxide In methanol at 50℃; for 3h; Inert atmosphere; Glovebox; Sealed tube; enantioselective reaction; | 92% |
-
-
6380-23-0
3,4-dimethoxystyrene

-
-
1593-60-8
p-toluenesulfonylhydroxylamine

-
-
1377813-97-2
1-(3,4-dimethoxyphenyl)-2-tosylethanone oxime

| Conditions | Yield |
|---|---|
| With tetrabutylammonium periodite In dichloromethane at -20 - 40℃; for 3h; Inert atmosphere; | 91% |
Benzene,4-ethenyl-1,2-dimethoxy- Specification
The Benzene, 4-ethenyl-1, 2-dimethoxy-, with the CAS registry number 6380-23-0, is also known as 3, 4-Dimethoxystyrene. It belongs to the product categories of Monomers; Polymer Science; Styrene and Functionalized Styrene Monomers. And its EINECS registry number is 228-962-9. This chemical's molecular formula is C10H12O2 and molecular weight is 164.2. What's more, its IUPAC name is 4-Ethenyl-1, 2-dimethoxybenzene. In addition, it must be stored in airtight containers at 2-8 °C.
Physical properties about Benzene, 4-ethenyl-1, 2-dimethoxy- are: (1)ACD/LogP: 2.53; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.53; (4)ACD/LogD (pH 7.4): 2.53; (5)ACD/BCF (pH 5.5): 49.02; (6)ACD/BCF (pH 7.4): 49.02; (7)ACD/KOC (pH 5.5): 564.37; (8)ACD/KOC (pH 7.4): 564.37; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 3; (12)Polar Surface Area: 18.46 Å2; (13)Index of Refraction: 1.53; (14)Molar Refractivity: 50.53 cm3; (15)Molar Volume: 163.3 cm3; (16)Polarizability: 20.03×10-24 cm3; (17)Surface Tension: 31 dyne/cm; (18)Density: 1.004 g/cm3; (19)Flash Point: 95.9 °C; (20)Enthalpy of Vaporization: 47.7 kJ/mol; (21)Boiling Point: 259.4 °C at 760 mmHg; (22)Vapour Pressure: 0.021 mmHg at 25 °C.
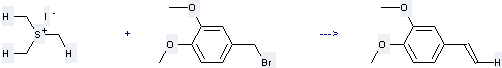
Preparation of Benzene, 4-ethenyl-1, 2-dimethoxy-: this chemical is prepared by reaction of 4-Bromomethyl-1, 2-dimethoxy-benzene with Trimethylsulfonium; iodide. This reaction needs reagents n-BuLi and LiI. Meanwhile, it needs solvents Tetrahydrofuran and Hexane. Other condition of this reaction is from 0 °C to room temp.. The yield is about 95 %.
Uses of Benzene, 4-ethenyl-1, 2-dimethoxy-: it is used to produce other chemicals. For example, it is used to produce 2-(3, 4-Dimethoxy-phenyl)-hexahydro-pyrrolo[1, 2-b]isoxazole by heating. The reaction needs solvent Toluene. The reaction time is 3 hours. The yield is about 88 %.
![Benzene, 4-ethenyl-1, 2-dimethoxy- can react with 3, 4-Dihydro-2H-pyrrole 1-oxide to get 2-(3, 4-Dimethoxy-phenyl)-hexahydro-pyrrolo[1, 2-b]isoxazole.](/UserFilesUpload/Uses of Benzene, 4-ethenyl-1, 2-dimethoxy-.png)
When you are using this chemical, please be cautious about it as the following:
As a chemical, it is irritating to eyes. In addition, this chemical may cause inflammation to the skin or other mucous membranes. During using it, wear suitable protective clothing and gloves. In case of contact with eyes, rinse immediately with plenty of water and seek medical advice.
You can still convert the following datas into molecular structure:
(1) SMILES: O(c1ccc(cc1OC)\C=C)C
(2) InChI: InChI=1/C10H12O2/c1-4-8-5-6-9(11-2)10(7-8)12-3/h4-7H,1H2,2-3H3
(3) InChIKey: NJXYTXADXSRFTJ-UHFFFAOYAG
Related Products
- Benzene
- Benzene, (10-bromodecyl)-
- Benzene,1,1',1'',1'''-(oxydimethylidyne)tetrakis-
- Benzene,1,1',1'',1'''-silanetetrayltetrakis-
- Benzene,1,1',1'',1'''-silanetetrayltetrakis[4-methyl-
- Benzene,1,1'-[1,2-bis(methylene)-1,2-ethanediyl]bis-
- Benzene,1,1'-(1,2-cyclopropanediyl)bis-
- Benzene,1,1'-(1,2-ethanediyl)bis(2,4-dinitro-)
- Benzene,1,1'-(1,2-ethanediyl)bis[2-methyl-
- Benzene,1,1'-(1,2-ethanediyl)bis[4-methyl-
- 638-02-8
- 63802-82-4
- 6380-28-5
- 638-04-0
- 638-07-3
- 63807-85-2
- 63808-36-6
- 638-08-4
- 6381-06-2
- 63810-78-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View