-
Name
Betulinic acid
- EINECS 207-448-8
- CAS No. 472-15-1
- Article Data59
- CAS DataBase
- Density 1.065 g/cm3
- Solubility
- Melting Point 295-298 °C (dec.)(lit.)
- Formula C30H48O3
- Boiling Point 550 °C at 760 mmHg
- Molecular Weight 456.709
- Flash Point 300.5 °C
- Transport Information
- Appearance powder
- Safety 24/25
- Risk Codes
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3-Hydroxylup-20(29)-en-28-oic acid;Lup-20(29)-en-28-oic acid, 3-hydroxy-, (3.beta.)-;Lup-20(29)-en-28-oic acid,3-hydroxy-,(3a)-;(1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Hydroxy-1-isopropenyl-5a,5b,8,8,11a-pentamethyl-eicosahydro-cyclopenta[a]chrysene-3a-carboxylic acid;Lup-20 (29)-en-28-oic acid, 3.beta.-hydroxy-;Mairin;Lup-20(29)-en-28-oic acid, 3-hydroxy-, (3beta)-;Prestwick_95;Lup-20 (29)-en-28-oic acid, 3-hydroxy-, (3.beta.)-;Lup-20(29)-en-28-oic acid, 3beta-hydroxy- (8CI);Betulic acid;RL9-080;Lup-20(29)-en-28-oic acid, 3-hydroxy-, (3β)-;
- PSA 57.53000
- LogP 7.08950
Synthetic route

| Conditions | Yield |
|---|---|
| Stage #1: betulonic acid With aluminum isopropoxide; benzyl alcohol In tetrahydrofuran at 65℃; for 2h; Stage #2: With sodium hydroxide In water; xylene at 130℃; for 1h; Stage #3: With acetic acid In water Conversion of starting material; | 96.3% |
| Stage #1: betulonic acid With aluminum isopropoxide; benzyl alcohol In tetrahydrofuran at 65℃; for 2h; Heating / reflux; Stage #2: With sodium hydroxide; water In xylenes at 130℃; for 1h; Stage #3: With acetic acid In water | 96.3% |
| With sodium tetrahydroborate; isopropyl alcohol; sodium hydroxide In water for 3h; | 94% |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran Acidic conditions; | 94% |
-
-
10376-50-8, 38736-81-1
3-O-acetylbetulinic acid

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 3h; Heating; | 89% |
| With potassium carbonate In methanol for 24h; | 88% |
| With potassium hydroxide | |
| With potassium hydroxide In methanol |

| Conditions | Yield |
|---|---|
| Stage #1: betulin With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene In acetic acid butyl ester; water; tert-butyl alcohol at 20℃; for 6h; Stage #2: With sodium chlorite; sodium dihydrogenphosphate; 2-methyl-but-2-ene In acetic acid butyl ester; water; tert-butyl alcohol Solvent; Concentration; Reagent/catalyst; | 89% |
| With 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; sodium chlorite; tetrabutylammomium bromide; sodium hypochlorite In phosphate buffer at 50℃; | 86% |
| With 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; tetrabutylammomium bromide; sodium hypochlorite In phosphate buffer at 50℃; pH=7.6; | 72% |
-
-
13159-28-9, 92594-07-5
betulinic aldehyde

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With sodium dihydrogenphosphate; sodium permanganate In dichloromethane; water; tert-butyl alcohol at 25℃; for 3h; | 85% |
| With sodium chlorite; sodium dihydrogenphosphate; 2-Methyl-1-butene; tert-butyl alcohol In toluene at 21℃; for 22h; Reagent/catalyst; Solvent; Temperature; Time; | 76% |
| With potassium permanganate In acetone at 0 - 20℃; | |
| With sodium chlorite; sodium dihydrogenphosphate In dichloromethane; tert-butyl alcohol |

| Conditions | Yield |
|---|---|
| With sodium tetrahydroborate In tetrahydrofuran Yields of byproduct given; | A 75% B n/a |
| Stage #1: betulonic acid With L-Selectride In tetrahydrofuran at -80℃; for 5h; Stage #2: With sodium hydroxide; dihydrogen peroxide In tetrahydrofuran for 1h; | A 19% B 38% |
| With sodium tetrahydroborate In tetrahydrofuran Yield given. Yields of byproduct given. Title compound not separated from byproducts; |

| Conditions | Yield |
|---|---|
| With synthetic air In 1,3,5-trimethyl-benzene at 140℃; under 760.051 Torr; for 6h; Catalytic behavior; | A 20% B 9% C 27% |
| With CeO2/TiO2; air In 1,3,5-trimethyl-benzene at 140℃; for 6h; Overall yield = 8 %; |

-
-
473-98-3
betulin

-
A
-
4439-98-9
betulonic aldehyde

-
B
-
13159-28-9, 92594-07-5
betulinic aldehyde

-
C
-
472-15-1
Betulinic acid

-
D
-
4481-62-3
betulonic acid

| Conditions | Yield |
|---|---|
| With dipyridinium dichromate In water; N,N-dimethyl-formamide | A 20% B 7% C 8% D 13% |


| Conditions | Yield |
|---|---|
| In 1,3,5-trimethyl-benzene at 140℃; for 4h; | A n/a B 5.3% |
-
-
135757-66-3
lup-20(29)-en-28-oic-3-O-β-D-glucopyranosyl (2->1)-O-β-D-glucopyranoside

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In methanol at 100℃; for 4h; |
-
-
80832-44-6
3β-trans-(3,4-dihydroxycinnamoyloxy)-20(29)-lupen-28-oic acid

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With potassium hydroxide In ethanol for 16h; Ambient temperature; | 220 mg |
-
-
75365-42-3
α-L-rhamnopyranosyl-3β-hydroxy-lup-20(29)-en-28-oic acid

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid for 5h; Heating; |

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In ethanol for 5h; Heating; |

-
A
-
2280-44-6
D-Glucose

-
B
-
73-34-7
L-rhamnose

-
C
-
532-20-7, 613-83-2, 7261-25-8, 7687-39-0, 13221-22-2, 14795-83-6, 15761-67-8, 20074-49-1, 25545-03-3, 25545-04-4, 32445-75-3, 34436-17-4, 36441-93-7, 36468-53-8, 37110-85-3, 37388-49-1, 38029-69-5, 40461-77-6, 40461-89-0, 41546-19-4, 41546-20-7, 41546-21-8, 41546-26-3, 41546-29-6, 41546-30-9, 41546-31-0, 126872-16-0, 131064-98-7
L-arabinose

-
D
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In water for 6h; Product distribution; Heating; |


-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With sulfuric acid In water for 6h; Heating; |
-
-
22333-85-3
28-O-β-D-glucopyranosyl 3β-hydroxy-lup-20(29)-en-28-oate

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With enzyme β-glucosidase In N,N-dimethyl-formamide at 37℃; for 72h; phosphate buffer (pH 5); |

| Conditions | Yield |
|---|---|
| Quantum yield; Product distribution; Further Variations:; Temperatures; Solvents; |

| Conditions | Yield |
|---|---|
| With potassium hydroxide In methanol for 3h; Heating; | A 1.8 mg B 3.1 mg |

-
A
-
3329-38-2
18-hydroxyoctadec-9-enoic acid

-
B
-
3233-92-9
9,10-epoxy-18-hydroxyoctadecanoic acid

-
D
-
506-45-6
22-hydroxydocosanoic acid

-
E
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water |


-
A
-
3329-38-2
18-hydroxyoctadec-9-enoic acid

-
B
-
3233-92-9
9,10-epoxy-18-hydroxyoctadecanoic acid

-
D
-
506-45-6
22-hydroxydocosanoic acid

-
E
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With hydrogenchloride In water |

-
-
27570-20-3
betulin monoacetate

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: tert-butyl chromate 2: KOH / methanol View Scheme | |
| Multi-step reaction with 2 steps 1: chromium (VI)-oxide; water containing acetic acid 2: ethanolic KOH-solution View Scheme | |
| Multi-step reaction with 2 steps 1: chromium (VI)-oxide; water containing acetic acid 2: ethanolic KOH-solution View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 80 percent / CrO3, H2SO4 / acetone / 1.5 h / 0 °C 2: 88 percent / aq. K2CO3 / methanol / 24 h View Scheme |
-
-
189571-52-6
3-hydroxy-28-[(tetrahydro-2H-pyran-2-yl)oxy]lup-20(29)-ene

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 87 percent / pyridine / 36 h / Ambient temperature 2: 95 percent / pyridinium p-toluene sulfonic acid salt / methanol / 36 h / Ambient temperature 3: 80 percent / CrO3, H2SO4 / acetone / 1.5 h / 0 °C 4: 88 percent / aq. K2CO3 / methanol / 24 h View Scheme |
-
-
189571-53-7
3-O-acetyl-28-O-tetrahydropyranylbetulin

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 95 percent / pyridinium p-toluene sulfonic acid salt / methanol / 36 h / Ambient temperature 2: 80 percent / CrO3, H2SO4 / acetone / 1.5 h / 0 °C 3: 88 percent / aq. K2CO3 / methanol / 24 h View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: benzene; KOH; ethanol 2: chromium (VI)-oxide; water containing acetic acid 3: ethanolic KOH-solution View Scheme | |
| Multi-step reaction with 3 steps 1: benzene; KOH; ethanol 2: chromium (VI)-oxide; water containing acetic acid 3: ethanolic KOH-solution View Scheme |
-
-
1125546-06-6
28-O-β-glucuronide betulinic acid

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With Escherichia coli β-D-glucuronidase at 37℃; for 1h; |

| Conditions | Yield |
|---|---|
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene In dichloromethane at 20℃; for 70h; | A n/a B 235 mg |
-
-
4439-98-9
betulonic aldehyde

-
-
13159-28-9, 92594-07-5
betulinic aldehyde

-
A
-
472-15-1
Betulinic acid

-
B
-
4481-62-3
betulonic acid

| Conditions | Yield |
|---|---|
| Stage #1: betulonic aldehyde; betulinic aldehyde With sodium chlorite; sodium dihydrogenphosphate; 2-methyl-but-2-ene In water; tert-butyl alcohol at 20℃; for 18.5h; Inert atmosphere; Stage #2: With sodium hydroxide In water; tert-butyl alcohol at 20℃; for 1.5h; Stage #3: With hydrogenchloride In water for 2.5h; Overall yield = 17.8 g; |


| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium chlorite; sodium dihydrogenphosphate; 2-methyl-but-2-ene / water; tert-butyl alcohol / 15.5 h / 20 °C / Inert atmosphere 1.2: 1.5 h / 20 °C 1.3: 2.5 h 2.1: sodium hydroxide; sodium tetrahydroborate / water; isopropyl alcohol / 3 h / Cooling with ice View Scheme | |
| Multi-step reaction with 2 steps 1.1: sodium chlorite; sodium dihydrogenphosphate; 2-methyl-but-2-ene / tert-butyl alcohol; water / 0.25 h 1.2: 1 h / Reflux 2.1: sodium tetrahydroborate / tetrahydrofuran View Scheme | |
| Multi-step reaction with 2 steps 1: sodium chlorite; dihydrogen peroxide; sodium dihydrogenphosphate / tert-butyl alcohol; water / 0.25 h / 0 - 10 °C 2: diisobutylaluminium hydride / dichloromethane; toluene / 1 h / 0 °C View Scheme |
-
-
472-15-1
Betulinic acid

-
-
18107-18-1
diazomethyl-trimethyl-silane

-
-
2259-06-5, 25493-95-2
methyl betulinate

| Conditions | Yield |
|---|---|
| In methanol; benzene | 100% |
| In methanol; toluene at 20℃; for 3h; Inert atmosphere; | 97% |
| In methanol at 20℃; for 2h; | 96% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 3h; Heating / reflux; | 100% |
| With potassium carbonate In N,N-dimethyl-formamide at 55℃; for 7h; | 85% |
| With potassium carbonate In N,N-dimethyl-formamide at 55℃; for 7h; | 84% |
| With potassium carbonate In N,N-dimethyl-formamide at 55℃; for 7h; | 84% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 4h; |
-
-
61798-18-3
4-methoxycarbonyl-3,3-dimethylbutyryl chloride

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In dichloromethane at 0 - 20℃; | 100% |
-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 2h; Stage #2: With ammonia In water; N,N-dimethyl-formamide at 20℃; for 16h; | 100% |
| Multi-step reaction with 3 steps 1.1: triethylamine; dmap; pyridine / 12 h / 25 °C 2.1: oxalyl dichloride / dichloromethane / 2 h / 25 °C / Cooling with ice 2.2: 1 h / 25 °C 3.1: potassium hydroxide / methanol; tetrahydrofuran / 24 h / 25 °C View Scheme |
-
-
472-15-1
Betulinic acid

-
-
100-39-0
benzyl bromide

-
-
192211-42-0
benzyl (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 60℃; for 3.5h; Inert atmosphere; | 99% |
| With potassium carbonate In acetone at 20℃; for 24h; | 99% |
| With potassium carbonate; caesium carbonate In acetone for 10h; Heating; | 98% |
-
-
472-15-1
Betulinic acid

-
-
212773-15-4
3-aminolup-20(29)-en-28-oic acid

| Conditions | Yield |
|---|---|
| With ammonium acetate; sodium cyanoborohydride In methanol for 40h; | 99% |
| Stage #1: Betulinic acid With ammonium acetate; sodium cyanoborohydride In methanol for 40h; Stage #2: With hydrogenchloride In methanol; water pH=2; Stage #3: With potassium hydroxide In water pH=10; | 99% |
| Multi-step reaction with 2 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide / 6 h / 20 °C 2: ammonium acetate; sodium cyanoborohydride / methanol / 12 h / 20 °C View Scheme | |
| Multi-step reaction with 2 steps 1: 1-hydroxy-3H-benz[d][1,2]iodoxole-1,3-dione / dimethyl sulfoxide; tetrahydrofuran / 6 h / 20 °C 2: ammonium acetate; sodium cyanoborohydride / methanol / 12 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: Jones reagent / acetone / 25 °C 2: ammonium acetate; sodium cyanoborohydride / methanol / 12 h / 25 °C View Scheme |
-
-
186581-53-3, 908094-01-9
diazomethane

-
-
472-15-1
Betulinic acid

-
-
2259-06-5, 25493-95-2
methyl betulinate

| Conditions | Yield |
|---|---|
| Stage #1: diazomethane; Betulinic acid In diethyl ether at 0 - 5℃; Stage #2: With acetic acid | 98% |
| In diethyl ether at 0 - 5℃; for 24h; | 90% |
| In diethyl ether; chloroform | 87% |
-
-
472-15-1
Betulinic acid

-
-
2845-62-7
benzenesulphonyl isocyanate

-
-
1187569-36-3
3-O-[N-(phenylsulfonyl)carbamoyl-17β-N-(phenylsulfonyl)amide]-betulinic acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 1h; Reflux; | 98% |
-
-
472-15-1
Betulinic acid

-
-
106-96-7
propargyl bromide

-
-
1400262-27-2
prop-2'-ynyl 3β-hydroxylup-20(29)-en-28-oate

| Conditions | Yield |
|---|---|
| With potassium carbonate In acetone for 14h; Reflux; | 98% |
| With potassium carbonate In acetone at 50℃; | 96% |
| With potassium carbonate In N,N-dimethyl-formamide at 20℃; for 16h; | 95% |

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With caesium carbonate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 6-bromo-hexanoic acid ethyl ester In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; | 98% |

| Conditions | Yield |
|---|---|
| With potassium carbonate In tetrahydrofuran at 50℃; | 98% |
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In dichloromethane; acetonitrile at 20℃; for 72h; | 72% |
| With potassium carbonate In N,N-dimethyl-formamide at 21℃; for 14h; |

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid; acetic anhydride With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 65℃; for 2h; Stage #2: With hydrogenchloride; water at 100℃; for 0.5h; | 97% |
| Stage #1: Betulinic acid; acetic anhydride With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 65℃; for 2h; Inert atmosphere; Stage #2: With hydrogenchloride; water at 100℃; for 0.5h; | 97% |
| With pyridine at 20℃; for 72h; | 96.2% |
-
-
472-15-1
Betulinic acid

-
-
102-92-1
Cinnamoyl chloride

-
-
267219-69-2
(1R,3aS,5aR,5bR,7aR,9R,11aR,11bR,13aR,13bR)-9-(cinnamoyloxy)-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylic acid

| Conditions | Yield |
|---|---|
| With dmap In benzene at 60℃; | 97% |
-
-
472-15-1
Betulinic acid

-
-
100-44-7
benzyl chloride

-
-
192211-42-0
benzyl (1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-hydroxy-5a,5b,8,8,11a-pentamethyl-1-(prop-1-en-2-yl)icosahydro-3aH-cyclopenta[a]chrysene-3a-carboxylate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide | 97% |
| With potassium carbonate In acetone at 20℃; for 20h; | 93% |
| With potassium carbonate In acetone at 20℃; for 20h; | 93% |
| With potassium carbonate In N,N-dimethyl-formamide | |
| With potassium carbonate In acetone |
-
-
34446-64-5, 42996-84-9
(E)-3-(4-methoxyphenyl)propenoyl chloride

-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With dmap In benzene at 60℃; | 96% |
-
-
472-15-1
Betulinic acid

-
-
79-04-9
chloroacetyl chloride

-
-
689279-93-4
(3S)-3-(2-chloroacetyloxy)-lup-20(29)-en-28-oic acid

| Conditions | Yield |
|---|---|
| With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; for 2h; Inert atmosphere; | 96% |
| With dmap; N-ethyl-N,N-diisopropylamine In tetrahydrofuran for 2h; Inert atmosphere; | 86% |
| With N-ethyl-N,N-diisopropylamine; dmap In tetrahydrofuran Inert atmosphere; | 86% |
| In N,N-dimethyl acetamide at 20℃; for 24h; |

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With caesium carbonate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 1-Bromo-3-phenylpropane In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; | 96% |
-
-
472-15-1
Betulinic acid

| Conditions | Yield |
|---|---|
| With aminosulfonic acid; urea In 1,4-dioxane; N,N-dimethyl-formamide at 65 - 75℃; | 96% |
-
-
472-15-1
Betulinic acid

-
-
32213-95-9
dimethyl L-aspartate hydrochloride

-
-
228419-37-2
(S)-2-[((1R,3aS,5aR,5bR,7aR,9S,11aR,11bR,13aR,13bR)-9-Hydroxy-1-isopropenyl-5a,5b,8,8,11a-pentamethyl-icosahydro-cyclopenta[a]chrysene-3a-carbonyl)-amino]-succinic acid dimethyl ester

| Conditions | Yield |
|---|---|
| With dmap; dicyclohexyl-carbodiimide In dichloromethane for 19h; | 95% |

| Conditions | Yield |
|---|---|
| With diazomethyl-trimethyl-silane In hexane; toluene at 20℃; | 95% |
| With Penicillium citreonigrum In chloroform; dimethyl sulfoxide Sonication; | 31.6 mg |
-
-
110-89-4
piperidine

-
-
472-15-1
Betulinic acid

-
-
96042-30-7
4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran

| Conditions | Yield |
|---|---|
| In chloroform | 95% |

-
-
472-15-1
Betulinic acid

-
-
2450-71-7
Propargylamine

-
-
1596376-96-3
N-propargyl-3β-hydroxy-lup-20(29)-en-28-amide

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium tetrafluoroborate; N-ethyl-N,N-diisopropylamine In tetrahydrofuran at 20℃; Stage #2: Propargylamine With potassium carbonate In N,N-dimethyl-formamide at 20℃; | 95% |
| Stage #1: Betulinic acid With benzotriazol-1-ol; N-ethyl-N,N-diisopropylamine; dicyclohexyl-carbodiimide In N,N-dimethyl-formamide at 20℃; for 0.5h; Inert atmosphere; Stage #2: Propargylamine In N,N-dimethyl-formamide at 20℃; for 12h; Inert atmosphere; | 76% |
| With benzotriazol-1-ol; dicyclohexyl-carbodiimide In tetrahydrofuran; dichloromethane at 0 - 20℃; for 25h; Inert atmosphere; | 51% |
-
-
472-15-1
Betulinic acid

-
-
123624-90-8
2-(chloromethyl)-3,5,6-trimethylpyrazine

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; Inert atmosphere; | 95% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; | 54.1% |
| With potassium carbonate In N,N-dimethyl-formamide at 25℃; for 12h; |
-
-
472-15-1
Betulinic acid

-
-
572-09-8
2,3,4,6-tetra-O-acetyl-α-D-glucopyranosyl bromide

-
-
22333-86-4
2,3,4,6-tetra-O-acetyl β-D-glucopyranosyl-3β-hydroxylup-20(29)-en-28-oate

| Conditions | Yield |
|---|---|
| With pyridine; silver(l) oxide for 3h; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride In N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: Cyclopropylamine With N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide at 20℃; | 94% |

| Conditions | Yield |
|---|---|
| Stage #1: Betulinic acid With caesium carbonate In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; for 0.5h; Stage #2: 1-Iodooctane In tetrahydrofuran; N,N-dimethyl-formamide at 20℃; | 94% |
-
-
472-15-1
Betulinic acid

-
-
92-95-5
4-biphenyl isocyanate

-
-
1187569-38-5
3-O-[N-(biphenyl)-p-carbamoyl]betulinic acid

| Conditions | Yield |
|---|---|
| With triethylamine In toluene for 1h; Reflux; | 93.5% |

| Conditions | Yield |
|---|---|
| With pyridinium chlorochromate In chloroform for 2h; Ambient temperature; | 93% |
| With Jones reagent In dichloromethane; acetone at 20℃; for 0.25h; Cooling; | 93% |
| With Jones reagent In dichloromethane; acetone at 20℃; Cooling; | 93% |
Betulinic acid Chemical Properties
The Molecular formula of Betulinic acid (CAS NO.472-15-1): C30H48O3
The Molecular Weight: 456.7
Molecular Structure : 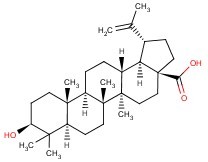
EINECS: 207-448-8
Synonyms of Betulinic acid (CAS NO.472-15-1): CCRIS 6748 ; Mairin ; NSC 113090 ; NSC 677578 ; 3-Hydroxylup-20(29)-en-28-oic acid ; Lup-20(29)-en-28-oic acid, 3-hydroxy-, (3beta)- ; Lup-20(29)-en-28-oic acid, 3beta-hydroxy- (8CI) Index of Refraction: 1.533
Molar Refractivity: 133.17 cm3
Molar Volume: 428.7 cm3
Polarizability: 52.79 10-24 cm3
Surface Tension: 39.9 dyne/cm
Density: 1.065 g/cm3
Flash Point: 300.5 °C
Enthalpy of Vaporization: 95.41 kJ/mol
Boiling Point: 550 °C at 760 mmHg
Vapour Pressure: 2.17E-14 mmHg at 25°C
refractive index: 7.8 ° (C=0.9, Pyridine)
Merck: 1190
IUPAC Name: 9-hydroxy-5b,8,8,11a-tetramethyl-1-prop-1-en-2-yl-2,3,4,5,5a,6,7,7a,9,10,11,11b,12,13,13a,13b-hexadecahydro-1H-cyclopenta[a]chrysene-3a-carboxylic acid
Synonyms: 3BETA-HYDROXY-20(29)-LUPENE-28-OIC ACID;3-B-HYDROXY-LUPA-20(30)-ENE 28-OIC ACID;3BETA-HYDROXY-20(29)-LUPAENE-28-OIC ACID;3B-HYDROXY-20(29)-LUPENE-28-OIC ACID;BETULINIC ACID;BETULIC ACID;MAIRIN;LUP-20(29)-EN-28-OIC ACID, 3-HYDROXY-, (3B)-;
Betulinic acid History
1.In 1995, Betulinic acid (CAS NO.472-15-1) was reported as a selective inhibitor of human melanoma.
Betulinic acid Uses
Betulinic acid (CAS NO.472-15-1) is a naturally occurring pentacyclic triterpenoid which has anti-retroviral, anti-malarial, and anti-inflammatory properties, as well as a more recently discovered potential as an anticancer agent, by inhibition of topoisomerase.
Betulinic acid Safety Profile
The Hazard Codes of Betulinic acid (CAS NO.472-15-1):  Xi
Xi
WGK Germany: 3
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View