-
Name
Bromisoval
- EINECS 207-825-7
- CAS No. 496-67-3
- Density 1.504 g/cm3
- Solubility 19.03g/L(temperature not stated)
- Melting Point 152 °C
- Formula C6H11BrN2O2
- Boiling Point
- Molecular Weight 223.07
- Flash Point
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Urea,(2-bromo-3-methylbutyryl)- (6CI,8CI);(RS)-2-Bromoisovalerylurea;(a-Bromoisovaleryl)urea;2-Bromo-3-methylbutyrylurea;2-Bromoisovalerylurea;Abroval;Alluval;Alural;BVU;Bromaral;Bromcarbamide;Bromisovalerylurea;Bromisovalum;Bromizoval;Bromocarbamide;Bromoisovalum;Bromovaleroylurea;Bromovalerylurea;Bromoxil;Bromuvan;Bromvalerylurea;Brovalin;Brovalurea;Calmotin;Dagrabromyl;Dormigene;Isobromyl;Pivadorm;Pivadorn;Upiol;a-Bromo-b-dimethylpropanoylurea;a-Bromoisovaleric acid ureide;a-Bromoisovaleroylurea;
- PSA 72.19000
- LogP 1.69200
Synthetic route
-
-
27109-47-3, 27318-02-1, 66067-58-1, 52574-82-0
2-bromoisovaleryl chloride

-
-
57-13-6
urea

-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| at 35 - 40℃; for 6h; Time; | 86% |
-
-
860762-74-9
N-(α-bromo-isovaleryl)-O-methyl-isourea

-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| With hydrogenchloride |

-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| With sodium hydroxide |

| Conditions | Yield |
|---|---|
| With Petroleum ether und Behandeln des entstandenen <α-Brom-isovaleryl>-isocyanats mit Ammoniak; |
-
-
7647-01-0
hydrogenchloride

-
-
860762-74-9
N-(α-bromo-isovaleryl)-O-methyl-isourea

-
A
-
74-87-3
methylene chloride

-
B
-
496-67-3
bromisoval

-
-
26464-05-1
2-bromo-3-methyl-butyryl bromide

-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: water; benzene; NaOH-solution 2: hydrochloric acid View Scheme | |
| Multi-step reaction with 2 steps 1: diethyl ether 2: hydrochloric acid View Scheme |

| Conditions | Yield |
|---|---|
| With water; zinc In acetonitrile at 80℃; for 7h; Sealed tube; Inert atmosphere; | 94% |
| With water; zinc In acetonitrile at 80℃; for 9h; Inert atmosphere; Sealed tube; | 94% |
| With sodium hydroxide; potassium dihydrogenphosphate; rat liver microsomes; NADPH In water at 37℃; Product distribution; other reagents; |
-
-
496-67-3
bromisoval

| Conditions | Yield |
|---|---|
| With water-d2; zinc In acetonitrile at 80℃; for 18h; Time; Inert atmosphere; Sealed tube; | 91% |
| With water-d2; zinc In acetonitrile at 80℃; for 18h; Inert atmosphere; Sealed tube; | 84% |

| Conditions | Yield |
|---|---|
| With butanone |
-
-
496-67-3
bromisoval

-
-
15900-26-2
2-amino-5-isopropyl-oxazol-4-one

| Conditions | Yield |
|---|---|
| With ammonium carbonate |

| Conditions | Yield |
|---|---|
| With 4-methyl-2-pentanone |
-
-
496-67-3
bromisoval

-
-
26843-84-5
2,4-diisopropyl-3-thia-glutaric acid-diureide

| Conditions | Yield |
|---|---|
| With potassium sulphide; ethanol | |
| With sodium sulfide In ethanol |
-
-
496-67-3
bromisoval

-
-
61226-21-9
Antipyrinsaeurechlorid

| Conditions | Yield |
|---|---|
| With sulfuric acid at 60℃; |

| Conditions | Yield |
|---|---|
| at 155℃; |
-
-
496-67-3
bromisoval

-
-
140-89-6
potassium ethyl xanthogenate

-
-
26843-82-3
Dithiocarbonic acid O-ethyl ester S-(2-methyl-1-ureidocarbonyl-propyl) ester

| Conditions | Yield |
|---|---|
| In ethanol |

| Conditions | Yield |
|---|---|
| In ethanol |
-
-
496-67-3
bromisoval

-
-
507-09-5
thioacetic acid

-
-
26843-85-6
Thioacetic acid S-(2-methyl-1-ureidocarbonyl-propyl) ester

| Conditions | Yield |
|---|---|
| With sodium hydroxide In ethanol |
-
-
496-67-3
bromisoval
-
-
496-67-3
bromisoval
Bromisoval Chemical Properties
IUPAC Name: Bromisoval [INN]
The MF of Bromisoval [INN] (496-67-3) is C6H11BrN2O2.
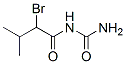
The MW of Bromisoval [INN] (496-67-3) is 223.07.
Synonyms of Bromisoval [INN] (496-67-3): Rarechem ax ki 5046 ; 1-(2-Bromoisovaleryl)urea ; A-(Bromoisovaleryl)urea ; Alpha(bromoisovaleryl)urea ; Labotest-bb lt00134620 ; Bromovalerylurea ; Bromoisovalerylurea ; Bromo-iso-valerylurea (alpha)
Index of Refraction: 1.514
EINECS: 207-825-7
Density: 1.504 g/ml
Melting point: 152 °C
Merck: 1397
Bromisoval Uses
Bromisoval [INN] (496-67-3) has a mild sedative and hypnotic effects, serving markedly after 5 minutes.Eeffect last for 4 hours, no habit.Can be made into powder, tablets and injections.
Bromisoval Production
With isoamyl alcohol as raw material, the first oxidation of isovalerate, and then brominated to α-bromo-isovaleryl bromide and finally generate the bromine and urea condensation of iso-amyl urea.
Bromisoval Toxicity Data With Reference
| 1. | orl-wmn TDLo:400 mg/kg:CNS | BMJOAE British Medical Journal. 1 (1955),1238. | ||
| 2. | orl-hmn LDLo:57 mg/kg | TOIZAG Toho Igakkai Zasshi. Journal of Medical Society of Toho University. 7 (1960),513. | ||
| 3. | orl-rat LD50:1000 mg/kg | FEPRA7 Federation Proceedings, Federation of American Societies for Experimental Biology. 7 (1948),262. | ||
| 4. | orl-mus LD50:2 g/kg | OYYAA2 Oyo Yakuri. Pharmacometrics. 11 (1976),693. | ||
| 5. | orl-cat LD50:450 mg/kg | NIIRDN “Drugs in Japan. Ethical Drugs, 6th Edition 1982“ Edited by Japan Pharmaceutical Information Center. 6 (1982),738. | ||
| 6. | orl-rbt LD50:1200 mg/kg | MEIEDD Merck Index. 10 (1983),193. |
Bromisoval Consensus Reports
Reported in EPA TSCA Inventory.
Bromisoval Safety Profile
Moderately toxic by ingestion. Human systemic effects by ingestion: nausea or vomiting, and coma. A sedative and hypnotic agent. When heated to decomposition it emits very toxic fumes of Br− and NOx.Safety information of Bromisoval [INN] (496-67-3):
RTECS YS3150000
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View