-
Name
Butyl butyrate
- EINECS 203-656-8
- CAS No. 109-21-7
- Article Data279
- CAS DataBase
- Density 0.8675 g/cm3
- Solubility Insoluble in water, but soluble in ethanol and ethyl ether
- Melting Point -92 °C
- Formula C8H16O2
- Boiling Point 165 °C at 760 mmHg
- Molecular Weight 144.214
- Flash Point 49.4 °C
- Transport Information UN 3272 3/PG 3
- Appearance clear colorless to pale yellowish liquid
- Safety 2
- Risk Codes 10
-
Molecular Structure
- Hazard Symbols R10:;
- Synonyms Butyl butanoate;n-Butyl n-butanoate;1-Butyl butyrate;n-Butyl butyrate;
- PSA 26.30000
- LogP 2.12980
Synthetic route

| Conditions | Yield |
|---|---|
| With [Al(H2O)6][MS]3 In cyclohexane for 1h; Reagent/catalyst; Dean-Stark; Reflux; | 100% |
| With Candida antarctica B lipase In 2,2,4-trimethylpentane at 40℃; for 3h; Enzymatic reaction; | 98% |
| With DOOl-AlCl3 superacid resin for 1.5h; Heating; | 97% |

| Conditions | Yield |
|---|---|
| With C29H44Cl2N2Ru; potassium tert-butylate In toluene for 15h; Reagent/catalyst; Time; Reflux; | 100% |
| Ru complex at 118℃; for 12h; Inert atmosphere; | 99% |
| With dihydrogen peroxide; bromine In dichloromethane; water at 20℃; for 2h; | 99% |

| Conditions | Yield |
|---|---|
| With trimethylaluminum; benzene-1,2-diol; isopropyl alcohol In dichloromethane at 20℃; for 2h; | 99% |
| With tris(bis(trimethylsilyl)amido)lanthanum(III) In hexane; pentane at -78 - 20℃; | 45% |
| With aluminum ethoxide |

| Conditions | Yield |
|---|---|
| With ruthenium(III) 2,4-pentanedionate at 25℃; for 1h; | 98% |
| CoCl2 In acetonitrile for 2h; Ambient temperature; | 89% |
| With dmap; triethylamine In dichloromethane | |
| In p-cymene at 50℃; Kinetics; Solvent; |

| Conditions | Yield |
|---|---|
| Stage #1: 1-Chloropropane With magnesium; 1,2-dibromomethane In toluene at 60℃; for 3h; Inert atmosphere; Stage #2: propoxycarbonyl chloride With aluminum (III) chloride; manganese(ll) chloride In toluene at 0 - 20℃; for 2.25h; Inert atmosphere; | 95% |

| Conditions | Yield |
|---|---|
| With butyryl iodide; bis(acetylacetonate)nickel(II) at 20℃; for 2h; | A 90% B 81% |

| Conditions | Yield |
|---|---|
| With triethylsilane; bis(acetylacetonate)nickel(II) at 20℃; for 2h; | A 90% B 81% |

| Conditions | Yield |
|---|---|
| With polyethylene glycol 400 at 65 - 70℃; for 3.5h; | 90% |
-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
-
4346-18-3
phenyl butanoate

-
A
-
53729-69-4, 21329-67-9
HCo(CO)(P(C6H5)3)3

-
B
-
91583-66-3
phenoxotris(triphenylphosphine)cobalt(I)

-
C
-
74-98-6
propane

-
D
-
109-21-7
butyl butyrate

-
E
-
7727-37-9
nitrogen

| Conditions | Yield |
|---|---|
| In toluene byproducts: H2; n-PrCO2Ph added to CoH(N2)(PPh3)3 in toluene in vac., reacted for 1 day at room temp.; liquid phase analysed by GLC; hexane added, ppt. filtered, washed with hexane, dried in vac., recrystd. from C6H6-hexane; | A n/a B 60% C 12% D 38% E 89% |


| Conditions | Yield |
|---|---|
| With rape seed lipase In hexane at 40℃; for 48h; Enzymatic reaction; | 88% |
| With Candida antarctica lipase B (Novozym 435); 1-butyl-3-methylimidazolium Tetrafluoroborate at 40℃; for 24h; Product distribution; Kinetics; Further Variations:; Reaction partners; Reagents; | |
| With Candida antartica lipase B at 100℃; Enzyme kinetics; Further Variations:; Reagents; Temperatures; |

| Conditions | Yield |
|---|---|
| With dihydridotetrakis(triphenylphosphine)ruthenium; water; 1-Phenylbut-1-en-3-one In 1,2-dimethoxyethane at 180℃; for 24h; Product distribution; Mechanism; in the absence of hydrogen acceptor (benzalacetone); other aldehydes; | A n/a B 85% C n/a |


| Conditions | Yield |
|---|---|
| With butyryl chloride; bis(acetylacetonate)nickel(II) at 90℃; for 2h; | A 83% B 68% |
-
-
29681-51-4
n-pentyldimethylsilane

-
A
-
109-21-7
butyl butyrate

-
B
-
25938-34-5
dimethylpentylsilylchloride

| Conditions | Yield |
|---|---|
| With butyryl chloride; bis(acetylacetonate)nickel(II) at 90℃; for 2h; | A 69% B 81% |

| Conditions | Yield |
|---|---|
| With butyryl chloride; bis(acetylacetonate)nickel(II) at 90℃; for 2h; | A 64% B 81% |
-
-
106-31-0
butanoic acid anhydride

-
-
21373-88-6, 16920-54-0
(PPh3)3CoH(N2)

-
A
-
99668-71-0
Co(OCO-n-C3H7)

-
B
-
74-98-6
propane

-
C
-
109-21-7
butyl butyrate

-
D
-
7727-37-9
nitrogen

-
E
-
1333-74-0
hydrogen

| Conditions | Yield |
|---|---|
| In toluene in toluene at room temp. for 1 day; | A n/a B 16% C 30% D 81% E 13% |


| Conditions | Yield |
|---|---|
| With triethylsilane; iron(III)-acetylacetonate at 20℃; | 80% |
-
-
27599-25-3, 54083-06-6, 19529-00-1
dihydridotetrakis(triphenylphosphine)ruthenium

-
-
123-72-8
butyraldehyde

-
A
-
25087-79-0
RuH(OCOC3H7)(P(C6H5)3)3

-
B
-
109-21-7
butyl butyrate

-
C
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| In neat (no solvent) educts mixed at in vac., stirred at -30°C for 2 h; evapd. in vac., solid washed with Et2O and hexane, dissolved in toluene, filtered, concd. in vac., ppt. filtered, washed with hexane, dried in vac.; | A 80% B n/a C <1 |
| In neat (no solvent) educts mixed at in vac., stirred at 0°C for 2 h; evapd. in vac., solid washed with Et2O and hexane, dissolved in toluene, filtered, concd. in vac., ppt. filtered, washed with hexane, dried in vac.; | A n/a B 0% C <1 |


| Conditions | Yield |
|---|---|
| With Bromotrichloromethane; [4,4’-bis(1,1-dimethylethyl)-2,2’-bipyridine-N1,N1‘]bis [3,5-difluoro-2-[5-(trifluoromethyl)-2-pyridinyl-N]phenyl-C]iridium(III) hexafluorophosphate at 20℃; for 12h; Irradiation; Inert atmosphere; | 80% |

| Conditions | Yield |
|---|---|
| With pyridine; 4-acetylamino-2,2,6,6-tetramethylpiperidine-N-oxyl; iodine; sodium hydrogencarbonate In dichloromethane; water at 20 - 25℃; for 3h; | A 79.7% B 20.3% |
| With sodium chlorate; sulfuric acid; vanadia | |
| With chromium(III) oxide; sulfuric acid |

| Conditions | Yield |
|---|---|
| In tetrachloromethane for 4h; Irradiation; | 78% |
| In benzene Ambient temperature; Irradiation; | 78% |

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite for 2h; Ambient temperature; | A 76% B 5% |
| With sodium bromate; sodium hydrogensulfite for 2h; Product distribution; Mechanism; Ambient temperature; other primary alcohols and α,ω-diols, var. solvents, temp. and time; | A 76% B 5% |
| With [pentamethylcyclopentadienyl*Ir(2,2′-bpyO)(OH)][Na]; sodium hydroxide In water for 40h; Time; Reflux; Inert atmosphere; | A 22% B 70% |

| Conditions | Yield |
|---|---|
| With diethylmethylsilane; bis(acetylacetonate)nickel(II) at 90℃; for 2h; | A 76% B 68% |
-
-
20266-12-0
1-butoxy-1-isobutoxy butane

-
A
-
78-83-1
2-methyl-propan-1-ol

-
B
-
109-21-7
butyl butyrate

-
C
-
539-90-2
isobutyl n-butyrate

-
D
-
79-31-2
isobutyric Acid

-
E
-
107-92-6
butyric acid

-
F
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With oxygen; cobalt(II) acetate at 90℃; under 750.06 Torr; Mechanism; Rate constant; other oxygen pressure; | A 5.5% B 3% C 3.5% D 5% E 73.5% F 7% |


| Conditions | Yield |
|---|---|
| With manganese (VII)-oxide In tetrachloromethane; acetone at -45℃; | 73% |
| With zinc dichromate(VI) In dichloromethane for 3h; Ambient temperature; | 60% |
| With sodium bromate; hydrogen bromide In dichloromethane at 35 - 40℃; for 20h; | 54% |
-
-
124-13-0
Octanal

-
-
71-36-3
butan-1-ol

-
A
-
109-21-7
butyl butyrate

-
B
-
124-07-2
Octanoic acid

-
C
-
589-75-3
butyl octanoate

| Conditions | Yield |
|---|---|
| With sodium bromate; sodium hydrogensulfite for 2h; Ambient temperature; | A 17% B 13% C 70% |

-
-
142-96-1
dibutyl ether

-
-
141-75-3
butyryl chloride

-
A
-
5455-24-3
octane-4,5-dione

-
B
-
109-21-7
butyl butyrate

| Conditions | Yield |
|---|---|
| CoCl2 In acetonitrile Ambient temperature; | A 10% B 68% |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; bromine In dichloromethane; water at 20℃; for 4h; | A 65% B 8% C 10% |

-
-
64-17-5
ethanol

-
-
123-73-9
crotonaldehyde

-
A
-
10544-63-5
ethyl crotonate

-
B
-
109-21-7
butyl butyrate

-
C
-
71-36-3
butan-1-ol

| Conditions | Yield |
|---|---|
| With sodium carbonate; rhodium(III) chloride; triphenylphosphine for 5h; Heating; | A 8 % Chromat. B 61% C 31% |


| Conditions | Yield |
|---|---|
| With ethanol; sodium carbonate; rhodium(III) chloride; triphenylphosphine for 5h; Heating; | A 8% B 61% C 31% |


| Conditions | Yield |
|---|---|
| With C30H34Cl2N2P2Ru; potassium methanolate; hydrogen In tetrahydrofuran at 100℃; under 38002.6 - 76005.1 Torr; for 15h; Glovebox; Autoclave; | 98% |
| With hydrogen at 223.84℃; under 5625.56 Torr; Kinetics; Thermodynamic data; Concentration; Pressure; Temperature; neat (no solvent, gas phase); | |
| With [RuH(η2-BH4)(2-di-tert-butylphosphinomethyl-6-diethylaminomethylpyridine)]; hydrogen In tetrahydrofuran at 110℃; under 7600.51 Torr; for 12h; Autoclave; | 97 %Chromat. |

| Conditions | Yield |
|---|---|
| With carbonylhydrido[6-(di-tert-butylphosphinomethylene)-2-(N,N-diethylaminomethyl)-1,6-dihydropyridine]ruthenium(II) In toluene; benzene at 135℃; for 24h; Inert atmosphere; | 97% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido[6-(di-tert-butylphosphinomethylene)-2-(N,N-diethylaminomethyl)-1,6-dihydropyridine]ruthenium(II) In toluene; benzene at 135℃; for 21h; Inert atmosphere; | 95% |
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); potassium tert-butylate In toluene at 120℃; for 48h; Inert atmosphere; Schlenk technique; Reflux; Green chemistry; | 55% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido[6-(di-tert-butylphosphinomethylene)-2-(N,N-diethylaminomethyl)-1,6-dihydropyridine]ruthenium(II) In toluene; benzene at 135℃; for 19h; Inert atmosphere; | 94% |
-
-
109-01-3
1-methyl-piperazine

-
-
109-21-7
butyl butyrate

-
-
10001-51-1
1-(4-methylpiperazin-1-yl)butan-1-one

| Conditions | Yield |
|---|---|
| With carbonylhydrido[6-(di-tert-butylphosphinomethylene)-2-(N,N-diethylaminomethyl)-1,6-dihydropyridine]ruthenium(II) In toluene; benzene at 135℃; for 24h; Inert atmosphere; | 94% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido[6-(di-tert-butylphosphinomethylene)-2-(N,N-diethylaminomethyl)-1,6-dihydropyridine]ruthenium(II) In toluene for 34h; Inert atmosphere; Reflux; | 92% |

| Conditions | Yield |
|---|---|
| With C31H40MnN2O3P*C6H14O; potassium tert-butylate In toluene; 1,3,5-trimethyl-benzene at 110℃; for 48h; Schlenk technique; | 88% |

| Conditions | Yield |
|---|---|
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; Barbier Coupling Reaction; chemoselective reaction; | 87% |
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; chemoselective reaction; | 87% |

| Conditions | Yield |
|---|---|
| With C31H40MnN2O3P*C6H14O; potassium tert-butylate In toluene; 1,3,5-trimethyl-benzene at 110℃; for 48h; Schlenk technique; | 85% |

| Conditions | Yield |
|---|---|
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; Barbier Coupling Reaction; chemoselective reaction; | 83% |
| With magnesium; copper(II) oxide In tetrahydrofuran at 65℃; for 4h; chemoselective reaction; | 83% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); potassium tert-butylate In toluene at 120℃; for 48h; Inert atmosphere; Schlenk technique; Reflux; Green chemistry; | 82% |

| Conditions | Yield |
|---|---|
| With carbonylhydrido(tetrahydroborato)[bis(2-diphenylphosphinoethyl)-amino]ruthenium(II); potassium tert-butylate In toluene at 120℃; for 48h; Inert atmosphere; Schlenk technique; Reflux; Green chemistry; | 80% |
Butyl butyrate Consensus Reports
Butyl butyrate Specification
The Butanoic acid, butyl ester, with the CAS registry number 109-21-7, is also known as n-Butyl n-butanoate. It belongs to the product categories of A-B Alphabetic; Alpha Sort; Chemical Class; Esters Analytical Standards; Solvents; Volatiles/ Semivolatiles; A-B; A-B Flavors and Fragrances; Alphabetical Listings; Certified Natural Products; Flavors and Fragrances; C8 to C9; Carbonyl Compounds; Esters. Its EINECS number is 203-656-8. This chemical's molecular formula is C8H16O2 and molecular weight is 144.21. What's more, its systematic name is butyl butanoate. Its classification code is Skin / Eye Irritant. This chemical is stable under normal temperatures and pressures. It should be sealed and stored in a cool and dry place. Moreover, it should be protected from oxides, bases, acids, heat and fire. It is a marine pollutant. It mildly irritates the eyes and skin. It is flammable and you should keep it out of the reach of children. Like other volatile esters, it has a pleasant aroma. It is used in the flavor industry to create sweet fruity flavors that are similar to that of pineapple. It occurs in many kinds of fruit including apple, banana, berries, pear, plum, and strawberry. It is used in organic synthesis. It can be used as solvents of nitrification cellulose, shellac, coumarone resin and coating.
Physical properties of Butanoic acid, butyl ester are: (1)ACD/LogP: 2.83; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.83; (4)ACD/LogD (pH 7.4): 2.83; (5)ACD/BCF (pH 5.5): 83.88; (6)ACD/BCF (pH 7.4): 83.88; (7)ACD/KOC (pH 5.5): 829.01; (8)ACD/KOC (pH 7.4): 829.01; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 6; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.412; (14)Molar Refractivity: 40.88 cm3; (15)Molar Volume: 164 cm3; (16)Polarizability: 16.2×10-24cm3; (17)Surface Tension: 27.3 dyne/cm; (18)Density: 0.878 g/cm3; (19)Flash Point: 49.4 °C; (20)Enthalpy of Vaporization: 40.15 kJ/mol; (21)Boiling Point: 165 °C at 760 mmHg; (22)Vapour Pressure: 1.91 mmHg at 25°C.
Preparation of Butanoic acid, butyl ester: this chemical can be prepared by butyraldehyde at the temperature of 20 °C. This reaction will need reagents catechol, Me3Al, iPrOH and solvent CH2Cl2 with the reaction time of 2 hours. The yield is about 99%.
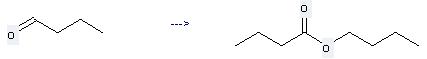
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCCCC)CCC
(2)Std. InChI: InChI=1S/C8H16O2/c1-3-5-7-10-8(9)6-4-2/h3-7H2,1-2H3
(3)Std. InChIKey: XUPYJHCZDLZNFP-UHFFFAOYSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 8900mg/kg (8900mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 521, 1979. | |
| rabbit | LD50 | oral | 9520mg/kg (9520mg/kg) | Industrial Medicine and Surgery. Vol. 41, Pg. 31, 1972. | |
| rabbit | LD50 | skin | > 5gm/kg (5000mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 521, 1979. | |
| rat | LD50 | intraperitoneal | 2300mg/kg (2300mg/kg) | Food and Cosmetics Toxicology. Vol. 17, Pg. 521, 1979. |
Related Products
- Butyl (R)-( )-2-(4-Hydroxyphenoxy)-propanoate
- Butyl benzoate
- Butyl benzyl phthalate
- Butyl beta-D-glucopyranoside
- Butyl bromoacetate
- Butyl butyrate
- Butyl butyryllactate
- Butyl carbobutoxymethyl phthalate
- Butyl hexanoate
- Butyl hydrogen phosphonate
- 109218-89-5
- 109220-81-7
- 109227-12-5
- 1092286-30-0
- 109229-22-3
- 109232-37-3
- 1092349-87-5
- 1092351-45-5
- 1092351-47-7
- 1092351-49-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View