Synthetic route
-
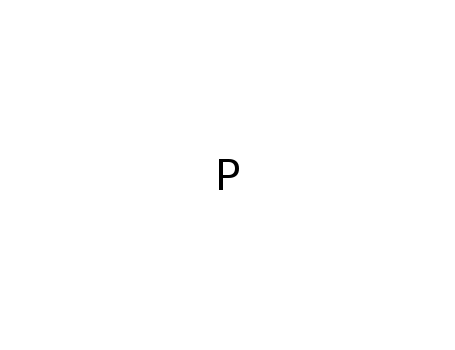
-
phosphorus
-

-
calcium oxide
Conditions
| Conditions | Yield |
|---|
| In water byproducts: PH3; addn. of 600 ml H2O to 120 g CaO, addn. of 20 g crushed, yellow P at boiling heat, boiling for 8-10 hours with replacement of vaporized H2O; introduction of CO2 into filtrate at 70-80°C; filtration after short boiling; vaporization till crystn.;; recrystn. from 3-4 parts H2O; yield 18-22 g pure Ca(PH2O2)2;; | |
| In water byproducts: PH3; addn. of 600 ml H2O to 120 g CaO, addn. of 20 g crushed, yellow P at boiling heat, boiling for 8-10 hours with replacement of vaporized H2O; introduction of CO2 into filtrate at 70-80°C; filtration after short boiling; vaporization till crystn.;; recrystn. from 3-4 parts H2O; yield 18-22 g pure Ca(PH2O2)2;; | |
-
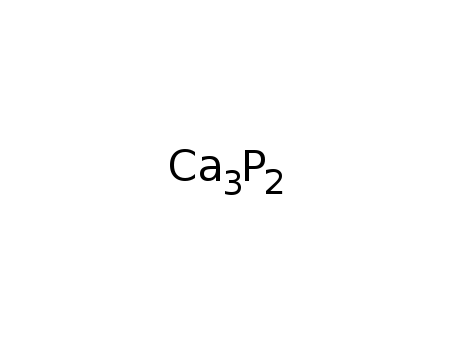
-
calcium phosphide
Conditions
| Conditions | Yield |
|---|
| With Ca2P2O7 In water decompn. of phosphorus lime with boiling water;; treatment of filtrate with CO2, crystn., drying at 100°C;; | |
| With Ca2P2O7 In water decompn. of phosphorus lime with boiling water;; treatment of filtrate with CO2, crystn., drying at 100°C;; | |
| In water | |
-

-
phosphorus
-
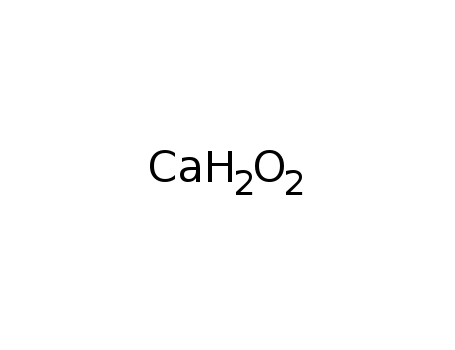
-
calcium hydroxide
Conditions
| Conditions | Yield |
|---|
| In chloroform; water byproducts: PH3; stirring on slight warming (ground temp. 44°C, surface temp. 37°C) for ca 80 hours; after storage for 24 hours decantation of clear layer, saturating with CO2, filtration;; vaporization in vac.;; | |
| In calcium hydroxide byproducts: PH3; addn. of small amt. of yellow P to boiling lime milk, till there is no formation of PH3;; removal of excessive Ca(OH)2 by introduction of CO2 at elevated temp. into filtrate; vaporization on air or over H2SO4 in vac.;; | |
| In water byproducts: PH3; aq. Ca(OH)2 suspension; in closed vessel;; | |
-
A
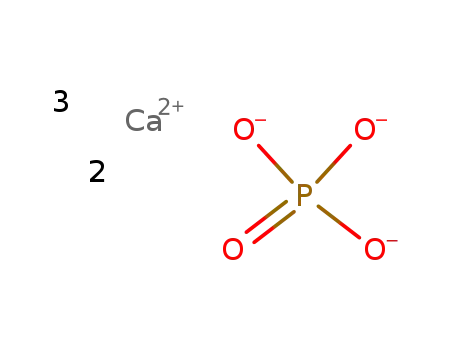
-
tricalcium diphosphate
Conditions
| Conditions | Yield |
|---|
| With calcium hydroxide In neat (no solvent) byproducts: H2; on boiling water bath; | |
Conditions
| Conditions | Yield |
|---|
| With calcium hydroxide In not given byproducts: H2; by heating; | |
-
-
dicalcium phosphate anhydrous
Conditions
| Conditions | Yield |
|---|
Stage #1: calcium bis(hypophosphite); calcium(II) nitrate In water
Stage #2: at 200 - 252℃; under 33098.3 Torr; for 0.733333h; | |
Conditions
| Conditions | Yield |
|---|
| With water without warming; | |
-
-
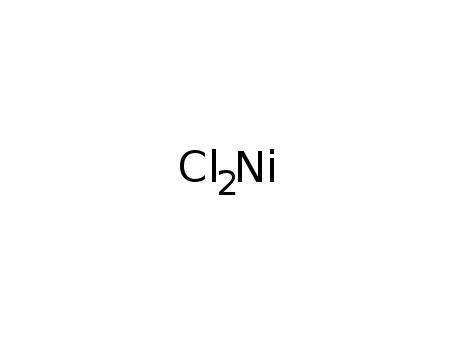
nickel dichloride
Conditions
| Conditions | Yield |
|---|
| With Na acetate In not given pH=5.0-5.5 (NaOH) , 92-93°C; | |
-
-
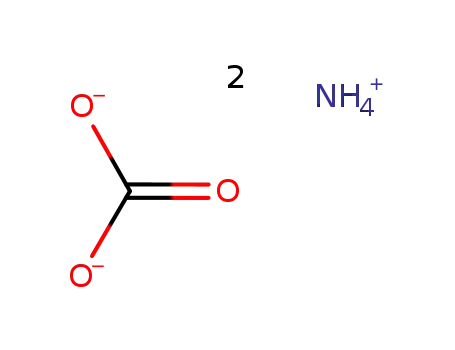
ammonium carbonate
Conditions
| Conditions | Yield |
|---|
| filtration, recrystn. from water or alc.; | |
-
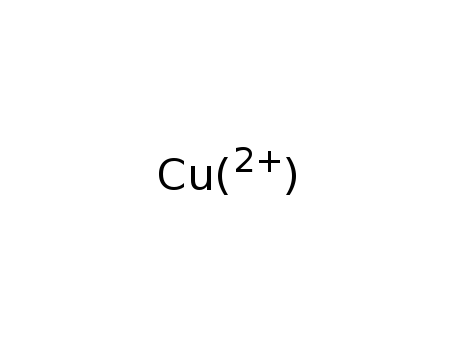
-
copper(II) ion
-
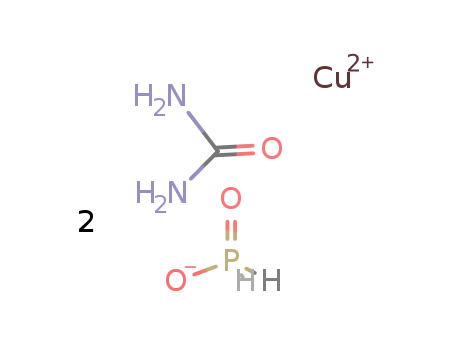
-
poly(copper(II)-di-μ-hypophosphito-μ-urea)
Conditions
| Conditions | Yield |
|---|
| In water Cu(II) : Ca(H2PO2)2 : (NH2)2CO = 1:1:2; evapn. of the soln. for several d at room temp.; elem. anal.; | |
-
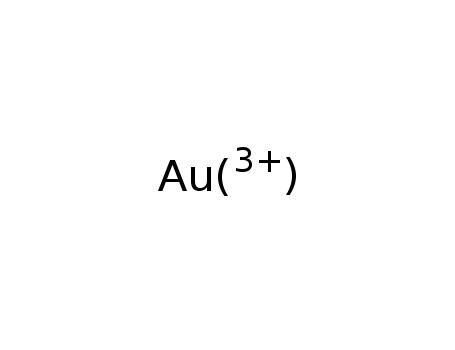
-
gold(III)
-
-
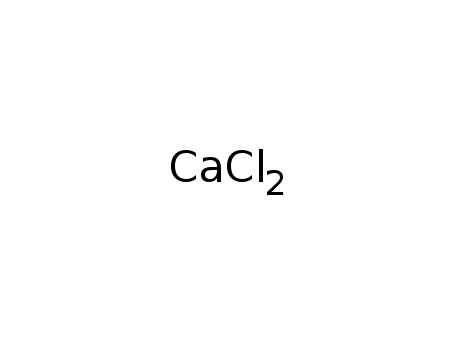
calcium chloride
-
B
-
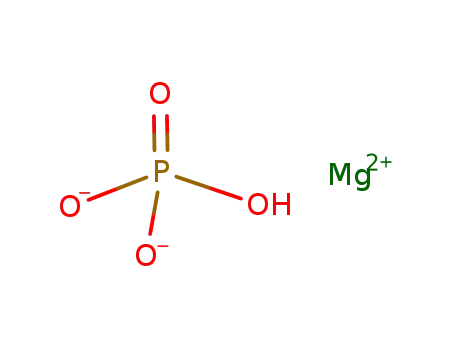
magnesium hydrogen phosphate
Conditions
| Conditions | Yield |
|---|
| In hydrogenchloride byproducts: CaCl2; introduction of a hydrochloric solution of Ca(H2PO4)2 in boiling aq. MgCl2;; | |
-
-
lead hypophosphite
-
-
Pb(2+)*H2PO2(1-)*NO3(1-)=PbH2PO2NO3
Conditions
| Conditions | Yield |
|---|
| filtration and evapn. of filtrate; product contaminated with Ca; | |
| filtration and evapn. of filtrate; product contaminated with Ca; | |
-

-
magnesium hydrogen phosphate
Conditions
| Conditions | Yield |
|---|
| In nitric acid introduction of a nitric solution of Ca(H2PO4)2 in boiling aq. Mg(NO3)2;; | |
| In nitric acid aq. HNO3; introduction of a nitric solution of Ca(H2PO4)2 in boiling aq. Mg(NO3)2;; | |
Conditions
| Conditions | Yield |
|---|
| In water byproducts: Ca-oxalate; addn. of a soln. of oxalic acid to a soln. of Ca(H2PO2)2; pptn. in coldness;; removal of Ca-oxalate impurities by heating at 100 °C, standing for 5 days and by repeated filtration;; | |
| In water byproducts: H3PO4, H3PO3; addn. of a hot soln. of 2 mol H2C2O4 in 100 ml H2O to a hot soln. of 2 mol Ca(H2PO2)2 in 2500 ml H2O (stirring); cooling, filtration, concentrating to a volume of 384 ml in a flow of N2 at 45 °C under reduced pressure;; content: 52 % H3PO2, less than 0.1 % H3PO3 and H3PO4;; | |
Conditions
| Conditions | Yield |
|---|
| In ethanol Ca(H2PO2)2 decompd. with Na2CO3 and alcoholic soln. evapd.;; | |
| In ethanol Ca(H2PO2)2 decompd. with Na2CO3 and alcoholic soln. evapd.;; | |
Conditions
| Conditions | Yield |
|---|
| In water byproducts: cadmium calcium phosphinite;; boiling an excess of Cd-oxalate with Ca-phosphinite in water; formation of crystals of cadmium calcium phosphinite by evaporation of the filtrate;; cadmium calcium phosphinite was not isolated; decomposition on heating under evolution of PH3;; | |
| In water byproducts: cadmium calcium phosphinite;; boiling an excess of Cd-oxalate with Ca-phosphinite in water; formation of crystals of cadmium calcium phosphinite by evaporation of the filtrate;; cadmium calcium phosphinite was not isolated; decomposition on heating under evolution of PH3;; | |
-
-
sodium carbonate decahydrate
Conditions
| Conditions | Yield |
|---|
| In water byproducts: CaCO3; addn. of 50g Ca(H2PO2)2 in 150ml H2O to a boiling soln. of 42g Na2CO3*10H2O in 100ml H2O, pptn. of CaCO3, filtration, evapn. of filtrate;; | |
| In water byproducts: CaCO3; addn. of 50g Ca(H2PO2)2 in 150ml H2O to a boiling soln. of 42g Na2CO3*10H2O in 100ml H2O, pptn. of CaCO3, filtration, evapn. of filtrate;; | |
-
-
ammonium sulfate
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) byproducts: P, calcium phosphate; calcination in retort; first small amt. of H2O and PH3, later sublimation of small amt. of P with formation of calcium phosphate;; | |
| In neat (no solvent) byproducts: P, calcium phosphate; calcination in retort; first small amt. of H2O and PH3, later sublimation of small amt. of P with formation of calcium phosphate;; | |
-
A
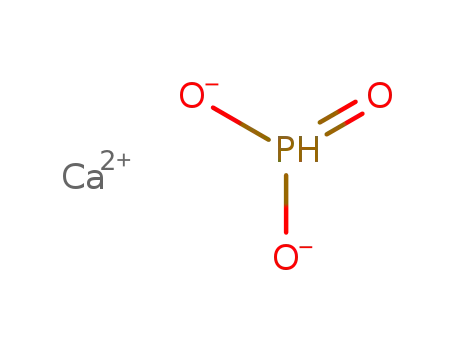
-
calcium phosphonate
Conditions
| Conditions | Yield |
|---|
| With sulfur dioxide In not given in acidic soln.; | |
| With SO2 In not given in acidic soln.; | |
-
A
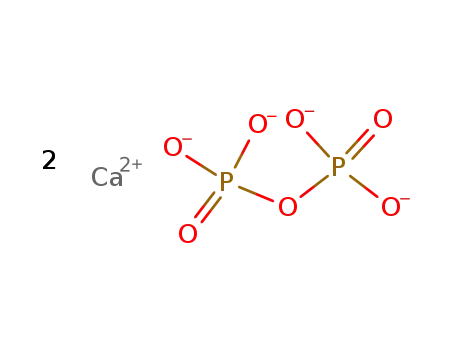
-
calcium pyrophosphate
-
B
-
calcium metaphosphate
-
-
phosphorous
Conditions
| Conditions | Yield |
|---|
| With hydrogen In neat (no solvent) heating in H2;; | |
| With H2 In neat (no solvent) heating in H2;; | |
Conditions
| Conditions | Yield |
|---|
| In neat (no solvent) decompn. by heating over 300°C;; | |
| In neat (no solvent) decompn. by heating over 300°C;; | |
Conditions
| Conditions | Yield |
|---|
| With sulfur dioxide In not given in neutral soln.; | |
| With SO2 In not given in neutral soln.; | |
-
-
magnesium hypophosphite * 6 H2O
Conditions
| Conditions | Yield |
|---|
| In not given boiling a solution of Ca(H2PO2)2 with an excess of a solution of Mg-oxalate; evaporation of the filtrate;; | |
| In not given boiling a solution of Ca(H2PO2)2 with an excess of a solution of Mg-oxalate; evaporation of the filtrate;; | |
Conditions
| Conditions | Yield |
|---|
| In water reaction of aq. soln. of Li2C2O4 and Ca(H2PO2)2 at 283-293 K; | |
-
-
cobalt(II) sulfate
-
-
hexaaquacobalt(II) bis(hypophosphite)
Conditions
| Conditions | Yield |
|---|
| In water slow evapn. aq. soln. of Co(II) hypophosphite, prepared by adding Ca(H2PO2)2 to CoSO4 (H2O);; mixt. filtered; crystals grown at 293 K in air; | |
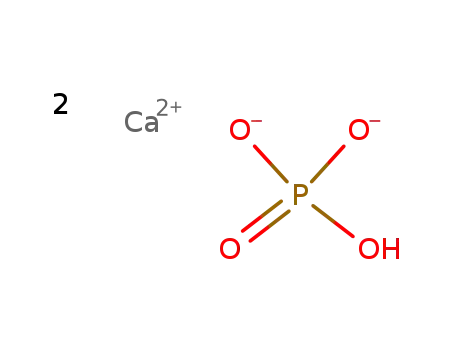
CALCIUM HYPOPHOSPHITE Chemical Properties
Product Name:Calcium hypophosphite(7789-79-9)
CAS No:7789-79-9
MF: CaH4O4P2
MW: 166.02
Sensitive : Hygroscopic
Calcium hypophosphite(7789-79-9)`s Synonyms: Phosphinicacid,calciumsalt;CALCIUM HYPOPHOSPHITE;CALCIUM PHOSPHINATE;CALCIUM HYPOPHOSPHITE EXTRA PURE, DAC;Lime hypophosphite;CALCIUM HYPOPHOSPHITE TECHNICAL;CALCIUMHYPOPHOSPHITE,PURIFIED;Calcium hydrophosphite
Calcium hypophosphite(7789-79-9)`s Molecular Structure:

CALCIUM HYPOPHOSPHITE Uses
As corrosion inhibitor, in nickel plating, Calcium hypophosphite has been used as dietary supplement.
CALCIUM HYPOPHOSPHITE Toxicity Data With Reference
Skin:Calcium hypophosphite may cause skin irritation or burns. Extravasation of iv infusions of calcium salts may cause cellulitis-type lesions.
Ingestion:Calcium hypophosphite is more irritating than other calcium salts.
CALCIUM HYPOPHOSPHITE Consensus Reports
First Aid Measures :
Ingestion: Seek medical attention. If individual is drowsy or unconscious, do not give anything by mouth; place individual on the left side with the head down. Contact a physician, medical facility, or poison control center for advice about whether to induce vomiting. If possible, do not leave individual unattended.
Inhalation: If symptoms develop, move individual away from exposure and into fresh air. If symptoms persist, seek medical attention. If breathing is difficult, administer oxygen. Keep person warm and quiet; seek immediate medical attention.
Skin:Remove contaminated clothing. Wash exposed area with soap and water. If symptoms persist, seek medical attention. Launder clothing before reuse.
Eyes:If symptoms develop, immediately move individual away from exposure and into fresh air. Flush eyes gently with water for at least 15 minutes while holding eyelids apart; seek immediate medical attention.
Storage:Keep in a cool, dry, dark location in a tightly sealed container or cylinder. Keep away from incompatible materials, ignition sources and untrained individuals. Secure and label area. Protect containers/cylinders from physical damage.
Handling:All chemicals should be considered hazardous. Avoid direct physical contact. Use appropriate, approved safety equipment. Untrained individuals should not handle this chemical or its container. Handling should occur in a chemical fume hood.
CALCIUM HYPOPHOSPHITE Safety Profile
Hazard Codes : F
Risk Statements : 11
Safety Statements :7-15
RIDADR : UN 3180 4.1/PG 2
WGK Germany : 3
CALCIUM HYPOPHOSPHITE Specification
Personal Protection:Chemical splash goggles in compliance with OSHA regulations are advised; however, OSHA regulations also permit other type safety glasses. Whre chemical resistant gloves. To prevent repeated or prolonged skin contact, wear impervious clothing and boots.
Fire Fighting:Wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear. During a fire, irritating and highly toxic gases may be generated by thermal decomposition or combustion. Use agent most appropriate to extinguish fire.
 This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
This product is a nationally controlled contraband, and the Lookchem platform doesn't provide relevant sales information.
 F
F






































