-
Name
[(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-β]furanyl Succinimidyl Carbonate
- EINECS 813-811-7
- CAS No. 253265-97-3
- Article Data17
- CAS DataBase
- Density 1.51 g/cm3
- Solubility
- Melting Point 122-125℃
- Formula C11H13 N O7
- Boiling Point 391.8 °C at 760 mmHg
- Molecular Weight 271.227
- Flash Point 190.7 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms 2,5-Pyrrolidinedione,1-[[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl]oxy]carbonyl]oxy]- (9CI)
- PSA 91.37000
- LogP -0.09710
Synthetic route
-
-
6066-82-6
1-hydroxy-pyrrolidine-2,5-dione

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In tetrahydrofuran for 2h; Reflux; | 99.6% |
-
-
74124-79-1
di(succinimido) carbonate

-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With pyridine In tert-butyl methyl ether at 25 - 40℃; for 62h; Solvent; | 90% |
| With triethylamine In acetonitrile at 23℃; for 7h; | 66% |
| With triethylamine In dichloromethane Solvent; Reagent/catalyst; Inert atmosphere; Reflux; Large scale; | 62% |
-
-
72605-53-9
γ-benzyloxymethyl-α,β-butenolide

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: benzophenone / 9 h / 0 °C / UV-irradiation 2: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 3: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 4: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 5: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 6: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 7: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 7 steps 1: benzophenone / 9 h / 0 °C / UV-irradiation 2: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 3: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 4: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 5: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 6: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 7: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
78508-96-0
(5S)-5-(hydroxymethyl)-5H-furan-2-one

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: benzophenone / 4 h / 0 °C / UV-irradiation 2: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 3: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 4: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 5: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 6: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 6 steps 1: benzophenone / 4 h / 0 °C / UV-irradiation 2: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 3: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 4: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 5: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 6: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
81703-94-8
methyl (2Z)-3-[(4S)-2,2-dimethyl-1,3-dioxolan-4-yl]acrylate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1: 97 percent / H2SO4 / methanol / 2 h / 23 °C 2: benzophenone / 4 h / 0 °C / UV-irradiation 3: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 4: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 5: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 6: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 7: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 7 steps 1: 97 percent / H2SO4 / methanol / 2 h / 23 °C 2: benzophenone / 4 h / 0 °C / UV-irradiation 3: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 4: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 5: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 6: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 7: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
140156-47-4
(4S,5S)-4-(1,3-dioxolan-2-yl)-5-hydroxymethyldihydrofuran-2-one

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 2: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 3: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 4: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 5: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 5 steps 1: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 2: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 3: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 4: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 5: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
186488-54-0
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl 4-nitrophenyl carbonate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 2: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
809286-93-9
(3aR,6aR)-3a,4,5,6a-tetrahydrofuro[2,3-b]furan-3(2H)-one

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 2: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
252873-50-0
(3S,3aS,6aR)-3-hydroxyhexahydrofuro[2,3-b]furan

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 2: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 3: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 3 steps 1: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 2: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 3: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
681463-02-5
(S)-1-(benzyloxy)but-3-en-2-yl acrylate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1: 98 percent / second generation Grubb's catalyst / CH2Cl2 / 5 h / Heating 2: benzophenone / 9 h / 0 °C / UV-irradiation 3: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 4: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 5: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 6: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 7: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 8: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 8 steps 1: 98 percent / second generation Grubb's catalyst / CH2Cl2 / 5 h / Heating 2: benzophenone / 9 h / 0 °C / UV-irradiation 3: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 4: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 5: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 6: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 7: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 8: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
681463-04-7
(2S,53)-3-(1,3-dioxolan-2-yl)pentane-1,2,5-triol

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 2: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 3: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 4: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 2: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 3: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 4: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
681463-03-6
(4S,5S)-5-(benzyloxymethyl)-4-(1,3-dioxolan-2-yl)dihydrofuran-2-one

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 2: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 3: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 4: triphenylphosphine; diisopropyl azodicarboxylate / benzene / 1.5 h / 23 °C 5: 326 mg / LiOH; Et3N / methanol; H2O / 2 h / 23 °C 6: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme | |
| Multi-step reaction with 6 steps 1: 89 percent / H2 / Pd/C / methanol / 24 h / 23 °C 2: LiAlH4 / tetrahydrofuran / 4 h / 0 °C 3: 145 mg / HCl / tetrahydrofuran; H2O / 40 h / 23 °C / pH 2 - 3 4: 94 percent / 4-methylmorpholino-N-oxide; tetrapropylammonium perruthenate / CH2Cl2 / 0.17 h / 23 °C 5: 70 percent / NaBH4 / ethanol / 2.5 h / -18 °C 6: 66 percent / Et3N / acetonitrile / 7 h / 23 °C View Scheme |
-
-
108-05-4
vinyl acetate

-
-
74124-79-1
di(succinimido) carbonate

-
-
109789-19-7
(3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
A
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: vinyl acetate; (3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol In dichloromethane; tert-butyl methyl ether at 20℃; for 5h; Resolution of racemate; Large scale reaction; Enzymatic reaction; Stage #2: di(succinimido) carbonate With triethylamine In acetonitrile at 20℃; for 24h; enantioselective reaction; | A 5.1 g B n/a |


-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: hydrogenchloride / water / 20 h / 2 - 5 °C 1.2: 1 h 2.1: triethylamine / acetonitrile / 5 h / 20 °C / Inert atmosphere View Scheme |
-
-
74124-79-1
di(succinimido) carbonate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 24 - 45℃; for 1h; |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: Lipase PS / 24 h / 50 °C / Enzymatic reaction 2: triethylamine / acetonitrile / 24 h / 20 °C / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: lipase AS / 24 h / 50 °C / Enzymatic reaction 2: triethylamine / acetonitrile / 24 h / 20 °C / Inert atmosphere View Scheme |
-
-
156928-09-5
(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-ol

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: triethylamine / dichloromethane / 3 h / 50 °C 2: triethylamine / tetrahydrofuran / 2 h / Reflux View Scheme |
-
-
109789-19-7
hexahydrofuro[2,3-b]furan-3-ol

-
-
74124-79-1
di(succinimido) carbonate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With pyridine In toluene at 40℃; for 9h; | 71.9 g |
-
-
36326-38-2, 64520-58-7, 91057-97-5, 91926-90-8, 104321-63-3, 124818-64-0, 128525-67-7, 128525-69-9, 104321-62-2
ethyl (E)-3-[(R)-2,2-dimethyl-1,3-dioxolan-4-yl]acrylate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 25 h / 0 - 30 °C 2.1: sodium tetrahydroborate; lithium bromide / tetrahydrofuran / 35 h / 0 - 30 °C 2.2: 0.33 h / 25 - 30 °C / Inert atmosphere 2.3: 5 h / 0 - 30 °C / Inert atmosphere 3.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme | |
| Multi-step reaction with 4 steps 1: 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 25 h / 0 - 30 °C 2: lithium bromide; lithium borohydride / tetrahydrofuran / 18 h / 0 - 30 °C / Inert atmosphere 3: sulfuric acid; sodium hydroxide / water / 2 h / 5 - 30 °C 4: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme | |
| Multi-step reaction with 5 steps 1.1: 1,8-diazabicyclo[5.4.0]undec-7-ene / acetonitrile / 25 h / 0 - 30 °C 2.1: methanol; hydrogenchloride / 3 h / 25 - 30 °C 3.1: sodium tetrahydroborate; methanol / tetrahydrofuran / 3 h / 65 - 70 °C 4.1: potassium tert-butylate / methanol / 0.17 h / 25 - 30 °C 4.2: 3 h / 0 - 30 °C 5.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: sodium tetrahydroborate; lithium bromide / tetrahydrofuran / 35 h / 0 - 30 °C 1.2: 0.33 h / 25 - 30 °C / Inert atmosphere 1.3: 5 h / 0 - 30 °C / Inert atmosphere 2.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme | |
| Multi-step reaction with 3 steps 1: lithium bromide; lithium borohydride / tetrahydrofuran / 18 h / 0 - 30 °C / Inert atmosphere 2: sulfuric acid; sodium hydroxide / water / 2 h / 5 - 30 °C 3: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme | |
| Multi-step reaction with 4 steps 1.1: methanol; hydrogenchloride / 3 h / 25 - 30 °C 2.1: sodium tetrahydroborate; methanol / tetrahydrofuran / 3 h / 65 - 70 °C 3.1: potassium tert-butylate / methanol / 0.17 h / 25 - 30 °C 3.2: 3 h / 0 - 30 °C 4.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: sodium tetrahydroborate; methanol / tetrahydrofuran / 3 h / 65 - 70 °C 2.1: potassium tert-butylate / methanol / 0.17 h / 25 - 30 °C 2.2: 3 h / 0 - 30 °C 3.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: potassium tert-butylate / methanol / 0.17 h / 25 - 30 °C 1.2: 3 h / 0 - 30 °C 2.1: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: sulfuric acid; sodium hydroxide / water / 2 h / 5 - 30 °C 2: pyridine / dichloromethane / 17 h / 40 - 45 °C View Scheme |
-
-
59256-86-9
ethyl 2-((3aR,5S,6R,6aR)-5-((R)-1,2-dihydroxyethyl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl)acetate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: sodium periodate / methanol / 2 h / 0 - 23 °C 2.1: sodium tetrahydroborate / methanol / 1 h / 0 °C 3.1: triethylamine / dichloromethane / 18 h / 0 - 23 °C / Inert atmosphere 4.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 4.2: 6 h / -78 - 23 °C 5.1: potassium carbonate / methanol / 0.25 h / 0 °C 6.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 7.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 8.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 9.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 9.2: 1.5 h / 0 °C 9.3: 1 h / 0 - 23 °C 10.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
178557-39-6
ethyl 2-[(3aR,5S,6R,6aR)-5-(hydroxymethyl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-yl]acetate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: triethylamine / dichloromethane / 18 h / 0 - 23 °C / Inert atmosphere 2.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 2.2: 6 h / -78 - 23 °C 3.1: potassium carbonate / methanol / 0.25 h / 0 °C 4.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 5.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 6.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 7.2: 1.5 h / 0 °C 7.3: 1 h / 0 - 23 °C 8.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
58399-68-1
(3aS,4S,6aR)-2-Oxo-hexahydro-furo[3,4-b]furan-4-carbaldehyde

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 2.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 3.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 3.2: 1.5 h / 0 °C 3.3: 1 h / 0 - 23 °C 4.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
57466-98-5
(Z)-ethyl 2-((3aR,5S,6aR)-5-((R)-2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethylfuro[2,3-d][1,3]dioxol-6(3aH,5H,6aH)-ylidene)acetate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: water; acetic acid / 60 h / 23 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 2.2: 6 h / -78 - 23 °C 3.1: potassium carbonate / methanol / 0.25 h / 0 °C 4.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 5.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 6.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 7.2: 1.5 h / 0 °C 7.3: 1 h / 0 - 23 °C 8.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
950583-17-2
(Z)-ethyl 2-((3aR,5S,6aR)-5-((R)-1,2-dihydroxyethyl)-2,2-dimethyltetrahydrofuro[2,3-d][1,3]dioxol-6-ylidene)acetate

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: palladium 10% on activated carbon; hydrogen / ethanol / 24 h / 760.05 Torr 2.1: sodium periodate / methanol / 2 h / 0 - 23 °C 3.1: sodium tetrahydroborate / methanol / 1 h / 0 °C 4.1: triethylamine / dichloromethane / 18 h / 0 - 23 °C / Inert atmosphere 5.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 5.2: 6 h / -78 - 23 °C 6.1: potassium carbonate / methanol / 0.25 h / 0 °C 7.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 8.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 9.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 10.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 10.2: 1.5 h / 0 °C 10.3: 1 h / 0 - 23 °C 11.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
1008743-57-4
3-deoxy-3-(2-ethoxy-2-oxoethyl)-1,2-O-isopropylidene-α-D-ribo-pentodialdo-1,4-furanose

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium tetrahydroborate / methanol / 1 h / 0 °C 2.1: triethylamine / dichloromethane / 18 h / 0 - 23 °C / Inert atmosphere 3.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 3.2: 6 h / -78 - 23 °C 4.1: potassium carbonate / methanol / 0.25 h / 0 °C 5.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 6.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 7.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 8.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 8.2: 1.5 h / 0 °C 8.3: 1 h / 0 - 23 °C 9.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Multi-step reaction with 8 steps 1.1: palladium 10% on activated carbon; hydrogen / ethanol / 6 h / 23 °C 2.1: boron trifluoride diethyl etherate / dichloromethane / 0.08 h / -78 °C 2.2: 6 h / -78 - 23 °C 3.1: potassium carbonate / methanol / 0.25 h / 0 °C 4.1: disodium hydrogenphosphate; Dess-Martin periodane / dichloromethane / 3 h / 0 - 23 °C 5.1: 3-chloro-benzenecarboperoxoic acid / dichloromethane / 2 h / 0 °C 6.1: hydrogenchloride / methanol / 12 h / 0 - 23 °C 7.1: lithium aluminium tetrahydride / tetrahydrofuran / 1 h / -78 - 23 °C / Inert atmosphere 7.2: 1.5 h / 0 °C 7.3: 1 h / 0 - 23 °C 8.1: triethylamine / acetonitrile / 16 h / 23 °C / Inert atmosphere View Scheme |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
156732-15-9
(2S,3S,5S)-2-(N,N-dibenzylamino)-3-hydroxy-5-amino-1,6-diphenylhexane

| Conditions | Yield |
|---|---|
| With N-ethyl-N,N-diisopropylamine In acetonitrile at 0 - 20℃; for 24h; | 100% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
251105-80-3
N-(3S-amino-2R-hydroxy-4-phenylbutyl)-N-isobutyl-4-nitro-benzenesulfonamide hydrochloride

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 30℃; for 6h; | 98.9% |
| With triethylamine In dichloromethane at 20℃; for 24h; Inert atmosphere; | 95% |
| With triethylamine In dichloromethane |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
865105-52-8
[(2R,3S)-3-[[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl]oxy]carbonyl]amino]-2-hydroxy-4-phenylbutyl](2-methylpropyl)carbamic acid phenylmethyl ester

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 24h; | 96.4% |
| With N-ethyl-N,N-diisopropylamine In dichloromethane | |
| With N-ethyl-N,N-diisopropylamine In dichloromethane for 0.5h; Inert atmosphere; | 5.8 g |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
865105-52-8
[(2R,3S)-3-[[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl]oxy]carbonyl]amino]-2-hydroxy-4-phenylbutyl](2-methylpropyl)carbamic acid phenylmethyl ester

| Conditions | Yield |
|---|---|
| Stage #1: 1-benzyl-3-(benzyloxycarbonyl-isobutyl-amino)-2-hydroxy-propyl-ammonium chloride; 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In dichloromethane at 20℃; for 24h; Stage #2: With sodium hydrogencarbonate In dichloromethane; water | 96.4% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
169280-56-2
4-amino-N-(2R,3S)(3-amino-2-hydroxy-4-phenylbutyl)-N-isobutylbenzenesulfonamide

| Conditions | Yield |
|---|---|
| In dimethyl sulfoxide at 15 - 30℃; Solvent; | 94% |
| With triethylamine In dichloromethane at 20℃; | 90% |
| With potassium carbonate In Isopropyl acetate; water at 15 - 35℃; for 4h; | 83.33% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
159006-03-8
tert-butyl (1S,2R)-1-benzyl-2-hydroxy-3-{isobutyl[(4-methoxyphenyl)sulfonyl]amino}propylcarbamate

-
-
206362-00-7
{(1S,2R)-1-Benzyl-2-hydroxy-3-[isobutyl-(4-methoxy-benzenesulfonyl)-amino]-propyl}-carbamic acid (3R,3aS,6aR)-(hexahydro-furo[2,3-b]furan-3-yl) ester

| Conditions | Yield |
|---|---|
| Stage #1: tert-butyl (1S,2R)-1-benzyl-2-hydroxy-3-{isobutyl[(4-methoxyphenyl)sulfonyl]amino}propylcarbamate With trifluoroacetic acid In dichloromethane at 23℃; for 0.666667h; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In dichloromethane at 23℃; for 3h; | 90% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 17h; | 89% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
183004-94-6
3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane

| Conditions | Yield |
|---|---|
| Stage #1: 3-S-(N-tert-butyloxyformamido)-[2R-hydroxy-1-[(4-aminophenylsulfonyl)(2-methylpropyl)]amino]-4-phenylbutane With trifluoroacetic acid In dichloromethane at 23℃; for 0.666667h; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In dichloromethane at 23℃; for 3h; | 89% |
-
-
160233-05-6
N-[(1S,2R)-3-[[(benzo-[1,3]-dioxole-5-sulfonyl)](isobutyl)amino]-1-benzyl-2-hydroxypropyl]carbamic acid tert-butyl ester

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: N-[(1S,2R)-3-[[(benzo-[1,3]-dioxole-5-sulfonyl)](isobutyl)amino]-1-benzyl-2-hydroxypropyl]carbamic acid tert-butyl ester With trifluoroacetic acid In dichloromethane at 20℃; for 1h; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In acetonitrile at 20℃; for 4h; Further stages.; | 82% |
| Stage #1: N-[(1S,2R)-3-[[(benzo-[1,3]-dioxole-5-sulfonyl)](isobutyl)amino]-1-benzyl-2-hydroxypropyl]carbamic acid tert-butyl ester With trifluoroacetic acid In dichloromethane at 20℃; for 1h; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In acetonitrile at 20℃; for 4h; | 82% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: C31H47BN2O7S With hydrogenchloride In 1,4-dioxane for 4h; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In dichloromethane at 20℃; Inert atmosphere; | 82% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: C30H45BN2O7S With hydrogenchloride In 1,4-dioxane at 22℃; under 760.051 Torr; for 4h; Inert atmosphere; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With triethylamine In dichloromethane at 22℃; under 760.051 Torr; for 16h; Inert atmosphere; | 82% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 48h; | 80% |
-
-
660410-38-8
2-Dimethylamino-benzothiazole-6-sulfonic acid ((2R,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-isobutyl-amide

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 78% |
-
-
473739-14-9
2-(2-Pyrrolidin-1-yl-ethylamino)-benzothiazole-6-sulfonic acid ((2R,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-isobutyl-amide

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 76% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In water; ethyl acetate at 25 - 35℃; | 73.19% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 73% |
-
-
1311412-72-2
C35H38ClFN4O4

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
1311410-46-4
C42H46ClFN4O8

| Conditions | Yield |
|---|---|
| In N,N-dimethyl-formamide at 20℃; for 1h; | 73% |
-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
622866-42-6
{(1S,2R)-[1-(4-formyl-benzyl)]-(2R)-2-hydroxy-3-[N-isobutyl-(N-4-methoxy-benzenesulfonyl)-amino]-propyl}-carbamic acid [3R,3aS,6aR]-hexahydrofuro[2,3-b]furan-3-yl ester

| Conditions | Yield |
|---|---|
| Stage #1: C27H38N2O7S With methanesulfonic acid In tetrahydrofuran at 40℃; Stage #2: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With 1-methyl-1H-imidazole In tetrahydrofuran at 50℃; Product distribution / selectivity; | 70% |
-
-
470704-95-1
2-(2-Dimethylamino-ethylamino)-benzooxazole-6-sulfonic acid ((2R,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-isobutyl-amide

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 67% |
-
-
1007876-78-9
C32H41N3O7S2

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| Stage #1: C32H41N3O7S2 With hydrogenchloride In 1,4-dioxane at 20℃; for 0.333333h; Stage #2: With N-ethyl-N,N-diisopropylamine In dichloromethane Stage #3: 1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione With N-ethyl-N,N-diisopropylamine | 67% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 66% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 64% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 40h; | 62% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 4h; | 60% |
-
-
473739-12-7
2-(2-Dimethylamino-ethylamino)-benzothiazole-6-sulfonic acid ((2R,3S)-3-amino-2-hydroxy-4-phenyl-butyl)-isobutyl-amide

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane at 20℃; for 3h; | 59% |
-
-
1217206-75-1
C24H33N3O2S

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

-
-
1217206-85-3
C31H41N3O6S

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane | 59% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 48h; | 59% |

-
-
253265-97-3
1-[[[(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yloxy]carbonyl]oxy]pyrrolidine-2,5-dione

| Conditions | Yield |
|---|---|
| With triethylamine In acetonitrile at 20℃; for 58h; | 58% |
Carbonic acid 2,5-dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl] ester Chemical Properties
Molecule structure of [(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-β]furanyl Succinimidyl Carbonate (CAS NO.253265-97-3):
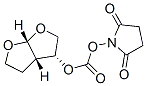
Molecular Weight: 271.2234 g/mol
Molecular Formula: C11H13NO7
Density: 1.51 g/cm3
Boiling Point: 391.8 °C at 760 mmHg
Flash Point: 190.7 °C
Index of Refraction: 1.562
Molar Refractivity: 57.98 cm3
Molar Volume: 178.5 cm3
Polarizability: 22.98×10-24 cm3
Surface Tension: 61 dyne/cm
Enthalpy of Vaporization: 64.14 kJ/mol
Vapour Pressure: 2.41E-06 mmHg at 25 °C
InChI: InChI=1/C11H13NO7/c13-8-1-2-9(14)12(8)19-11(15)18-7-5-17-10-6(7)3-4-16-10/h6-7,10H,1-5H2/t6-,7-,10+/m0/s1
InChIKey: VCFNCYVHQSHFRH-MHYGZLNHBY
Product Categories: Chiral Reagents; Heterocycles; Intermediates
Carbonic acid 2,5-dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl] ester Uses
[(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-β]furanyl Succinimidyl Carbonate (CAS NO.253265-97-3) is used as darunavir intermediate.
Carbonic acid 2,5-dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl] ester Specification
[(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-β]furanyl Succinimidyl Carbonate (CAS NO.253265-97-3) is also named as 1-[[[[(3R,3aS,6aR)-Hexahydrofuro[2,3-β]furan-3-yl]oxy]carbonyl]oxy]-2,5-pyrrolidinedione ; Carbonic Acid 2,5-Dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-Hexahydrofuro[2,3-β]furan-3-yl] Ester ; [(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-b]furanyl Succinimidyl Carbonate ; 1-[[[[(3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl]oxy]carbonyl]oxy]-2,5-pyrrolidinedione;Carbonic Acid 2,5-Dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-Hexahydrofuro[2,3-b]furan-3-yl] Ester . [(3R,3aS,6aR)-Hydroxyhexahydrofuro[2,3-β]furanyl Succinimidyl Carbonate (CAS NO.253265-97-3) is pale-yellow solid.
Related Products
- Carbonic acid
- Carbonic acid 2,5-dioxo-1-pyrrolidinyl [(3R,3aS,6aR)-hexahydrofuro[2,3-b]furan-3-yl] ester
- Carbonic acid 3-(p-iodophenyl)-3-methylpropyl isopropyl ester
- Carbonic acid 4-chloro-5-iodo-2-methylphenyl ethyl ester
- Carbonic acid 5-chloro-8-quinolyl ethyl ester
- Carbonic acid ammonium zirconium salt
- Carbonic acid bis(2-methylallyl) ester
- Carbonic acid di-2-pyridyl ester
- Carbonic acid, 2-methylene-1,3-propanediyl bis(1,1-dimethylethyl) ester
- Carbonic acid, barium salt
- 25327-89-3
- 253315-22-9
- 25332-13-2
- 25332-20-1
- 25332-39-2
- 253324-92-4
- 253332-82-0
- 25333-42-0
- 25333-81-7
- 253340-48-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View