-
Name
Carisoprodol
- EINECS 201-118-7
- CAS No. 78-44-4
- Article Data9
- CAS DataBase
- Density 1.056 g/cm3
- Solubility slightly soluble in water and freely soluble in alcohol, chloroform and acetone
- Melting Point 92-92 oC
- Formula C12H24N2O4
- Boiling Point 423.412 °C at 760 mmHg
- Molecular Weight 260.334
- Flash Point 209.872 °C
- Transport Information
- Appearance White powder.
- Safety 36
- Risk Codes 22
-
Molecular Structure
-
Hazard Symbols
 Xn
Xn
- Synonyms Carbamicacid, (1-methylethyl)-, 2-[[(aminocarbonyl)oxy]methyl]-2-methylpentyl ester(9CI);Carbamic acid, isopropyl-, 2-(hydroxymethyl)-2-methylpentyl estercarbamate (ester) (8CI);Carbamic acid, isopropyl-,2-(hydroxymethyl)-2-methylpentyl ester, carbamate (6CI);2-Methyl-2-propyl-1,3-propanediol carbamate isopropylcarbamate;Apesan;Atonalyt;Caprodat;Carisoma;Carisoprodate;Carisoprodatum;Domarax;Flexal;Isobamate;Isomeprobamate;Isoprotane;Isoprothane;Izoprotan;Mioril;N-Isopropyl-2-methyl-2-propyl-1,3-propanediol dicarbamate;NSC172124;Rela;Sanoma;Soma;Somalgit;Carisoprodol;
- PSA 90.65000
- LogP 3.11390
Synthetic route
-
-
25462-17-3
N-Mono-isopropyl-2-methyl-2-n-propylpropane-1,3-diol carbamate

-
-
3019-71-4
Trichloroacetyl isocyanate

-
-
78-44-4
carisoprodol

| Conditions | Yield |
|---|---|
| Stage #1: N-Mono-isopropyl-2-methyl-2-n-propylpropane-1,3-diol carbamate; Trichloroacetyl isocyanate In methanol; dichloromethane at 0 - 25℃; for 4h; Stage #2: With potassium carbonate In methanol; dichloromethane at 25℃; for 4h; | 96% |
-
-
25462-17-3
N-Mono-isopropyl-2-methyl-2-n-propylpropane-1,3-diol carbamate

-
-
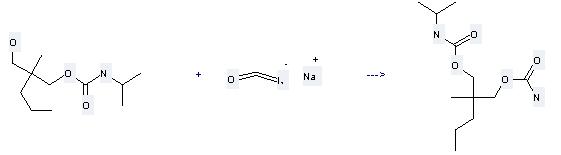
917-61-3
sodium isocyanate

-
-
78-44-4
carisoprodol

| Conditions | Yield |
|---|---|
| With hydrogenchloride In dichloromethane at -2 - 2℃; for 10h; | 80% |
| Stage #1: N-Mono-isopropyl-2-methyl-2-n-propylpropane-1,3-diol carbamate; sodium isocyanate With hydrogenchloride In dichloromethane at 0℃; for 2.5h; Stage #2: With sodium hydrogencarbonate In dichloromethane pH=8; |
-
-
75-44-5
phosgene

-
-
25462-17-3
N-Mono-isopropyl-2-methyl-2-n-propylpropane-1,3-diol carbamate

-
-
78-44-4
carisoprodol

| Conditions | Yield |
|---|---|
| (i) PhNMe2, (ii) NH3; Multistep reaction; |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: 5 h / Heating 2: 80 percent / HCl / CH2Cl2 / 10 h / -2 - 2 °C View Scheme | |
| Multi-step reaction with 2 steps 2: (i) PhNMe2, (ii) NH3 View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: 1.) Na / 1.) toluene, 90 deg C, 30 min, 2.) toluene, 88-110 deg C 2: 5 h / Heating 3: 80 percent / HCl / CH2Cl2 / 10 h / -2 - 2 °C View Scheme | |
| Multi-step reaction with 3 steps 1.1: p-toluenesulfonyl chloride; n-butyllithium 1.2: 0 - 60 °C 2.1: C49H74AlNO4; bis(triphenylphosphoranylidene)ammonium iodide / butanone / 80 h / 100 °C / 7500.75 Torr / Autoclave; Sealed tube 3.1: dichloromethane; methanol / 4 h / 0 - 25 °C 3.2: 4 h / 25 °C View Scheme |
-
-
13911-97-2
3-methyl 3-n-propyl oxetane

-
-
78-44-4
carisoprodol

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1.1: C49H74AlNO4; bis(triphenylphosphoranylidene)ammonium iodide / butanone / 80 h / 100 °C / 7500.75 Torr / Autoclave; Sealed tube 2.1: dichloromethane; methanol / 4 h / 0 - 25 °C 2.2: 4 h / 25 °C View Scheme |

| Conditions | Yield |
|---|---|
| With di-tert-butyl peroxide; copper diacetate; 4,4'-di-tert-butyl-2,2'-bipyridine In acetonitrile at 25℃; for 12h; Inert atmosphere; Irradiation; chemoselective reaction; | 60% |
-
-
78-44-4
carisoprodol

-
-
123-62-6
propionic acid anhydride

-
-
25648-91-3
N-Isopropyl-N'-propionyl-2-methyl-2-propyl-1,3-dicarbamoyloxy-propan

| Conditions | Yield |
|---|---|
| With sulfuric acid |
-
-
50-00-0
formaldehyd

-
-
78-44-4
carisoprodol

-
-
109-89-7
diethylamine

-
-
25648-95-7
N-Isopropyl-N'-diaethylamino-methyl-2-methyl-2-propyl-1,3-dicarbamoyloxy-propan

| Conditions | Yield |
|---|---|
| (i), (ii) /BRN= 605268/; Multistep reaction; |

-
-
78-44-4
carisoprodol

| Conditions | Yield |
|---|---|
| In aq. phosphate buffer at 24.84℃; pH=7.4; Thermodynamic data; Temperature; |
Carisoprodol Specification
The Carisoprodol, with the CAS registry number 78-44-4, is also known as N-Isopropyl 2-methyl-2-propyl-1,3-propanediol dicarbamate. It belongs to the product categories of Active Pharmaceutical Ingredients; API intermediates; Isotope Labeled Compounds; Intermediates & Fine Chemicals; Pharmaceuticals; Muscle Relaxants; Neurobiology; Pharmacologicals; Aliphatics; Amines. Its EINECS number is 201-118-7. This chemical's molecular formula is C12H24N2O4 and molecular weight is 260.33. What's more, its systematic name is 2-[(Carbamoyloxy)methyl]-2-methylpentyl isopropylcarbamate. Its classification codes are: (1)Central Nervous System Agents; (2)Drug / Therapeutic Agent; (3)Muscle relaxants, central; (4)Neuromuscular Agents; (5)Peripheral Nervous System Agents; (6)Relaxant [skeletal muscle]; (7)Reproductive Effect; (8)Tumor data. This chemical is harmful if swallowed. When using it, you need wear suitable protective clothing. It is a centrally acting skeletal muscle relaxant whose mechanism of action is not completely understood but may be related to its sedative actions. It is used as an adjunct in the symptomatic treatment of musculoskeletal conditions associated with painful muscle spasm.
Physical properties of Carisoprodol are:
(1)ACD/LogP: 2.099; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): 2.10; (4)ACD/LogD (pH 7.4): 2.10; (5)ACD/BCF (pH 5.5): 23.18; (6)ACD/BCF (pH 7.4): 23.18; (7)ACD/KOC (pH 5.5): 330.23; (8)ACD/KOC (pH 7.4): 330.23; (9)#H bond acceptors: 6; (10)#H bond donors: 3; (11)#Freely Rotating Bonds: 9; (12)Polar Surface Area: 90.65 Å2; (13)Index of Refraction: 1.466; (14)Molar Refractivity: 68.247 cm3; (15)Molar Volume: 246.499 cm3; (16)Polarizability: 27.055×10-24cm3; (17)Surface Tension: 37.14 dyne/cm; (18)Density: 1.056 g/cm3; (19)Flash Point: 209.872 °C; (20)Enthalpy of Vaporization: 67.774 kJ/mol; (21)Boiling Point: 423.412 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
Preparation of Carisoprodol:
Carisoprodol can be prepared by 2-(hydroxymethyl)-2-methylpentyl isopropyl-carbamate and cyanic acid; sodium cyanate at the temperature of -2 - 2 °C. This reaction will need regent HCl and solvent CH2Cl2 with the reaction time of 10 hours. The yield is about 80%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(OCC(COC(=O)NC(C)C)(C)CCC)N
(2)Std. InChI: InChI=1S/C12H24N2O4/c1-5-6-12(4,7-17-10(13)15)8-18-11(16)14-9(2)3/h9H,5-8H2,1-4H3,(H2,13,15)(H,14,16)
(3)Std. InChIKey: OFZCIYFFPZCNJE-UHFFFAOYSA-N
The toxicity data of Carisoprodol is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 800mg/kg (800mg/kg) | Naunyn-Schmiedeberg's Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 238, Pg. 92, 1960. | |
| mouse | LD50 | intravenous | 165mg/kg (165mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES | Journal of Pharmacology and Experimental Therapeutics. Vol. 127, Pg. 66, 1959. |
| mouse | LD50 | oral | 1800mg/kg (1800mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: CONVULSIONS OR EFFECT ON SEIZURE THRESHOLD | Arzneimittel-Forschung. Drug Research. Vol. 12, Pg. 340, 1962. |
| rabbit | LD50 | intravenous | 124mg/kg (124mg/kg) | SENSE ORGANS AND SPECIAL SENSES: OTHER: EYE BEHAVIORAL: REGIDITY BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) | International Journal of Neuropharmacology. Vol. 5, Pg. 305, 1966. |
| rat | LD50 | intraperitoneal | 450mg/kg (450mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE | Journal of Pharmacology and Experimental Therapeutics. Vol. 127, Pg. 66, 1959. |
| rat | LD50 | intravenous | 450mg/kg (450mg/kg) | Psychopharmacology Service Center, Bulletin. Vol. 2, Pg. 17, 1963. | |
| rat | LD50 | oral | 1320mg/kg (1320mg/kg) | LUNGS, THORAX, OR RESPIRATION: OTHER CHANGES BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) PERIPHERAL NERVE AND SENSATION: SPASTIC PARALYSIS WITH OR WITHOUT SENSORY CHANGE | Journal of Pharmacology and Experimental Therapeutics. Vol. 127, Pg. 66, 1959. |
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View