-
Name
Cholesteryl benzoate
- EINECS 210-064-3
- CAS No. 604-32-0
- Article Data51
- CAS DataBase
- Density 1.04 g/cm3
- Solubility INSOLUBLE
- Melting Point 148-150 °C(lit.)
- Formula C34H50O2
- Boiling Point 563.6 °C at 760 mmHg
- Molecular Weight 490.77
- Flash Point 213.1 °C
- Transport Information
- Appearance white powder
- Safety 22-24/25
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Cholest-5-en-3-ol(3b)-, benzoate (9CI);3b-(Benzoyloxy)cholest-5-ene;Benzoate cholesterol;(3β)-Cholest-5-en-3-yl benzoate;Cholest-5-en-3b-yl benzoate;Cholesterol 3b-benzoate;(3S,8S,9S,10R,13R,14S,17R)-10,13-Dimethyl-17-[(2R)-6-methyl-2-heptanyl]-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta[a]phenanthren-3-yl benzoate;
- PSA 26.30000
- LogP 9.25340
Synthetic route

| Conditions | Yield |
|---|---|
| With iron(III)-acetylacetonate In n-heptane at 105℃; for 30h; Inert atmosphere; | 97% |
| With tetramethylammonium methyl carbonate In hexane for 16h; Solvent; Molecular sieve; Reflux; Green chemistry; | 94% |
| With lanthanum(III) isopropoxide; 2-(2-methoxyethoxy)ethyl alcohol In hexane Reflux; chemoselective reaction; | 78% |
-
-
57-88-5
cholesterol

-
-
85909-02-0
N-benzyl-N-(tert-butoxycarbonyl)-benzamide

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With sodium t-butanolate In toluene at 150℃; for 24h; Sealed tube; | 96% |

| Conditions | Yield |
|---|---|
| With 1,8-diazabicyclo[5.4.0]undec-7-ene In acetonitrile for 4h; Ambient temperature; | 95% |
-
-
1416720-35-8
cholester-3-yl 4-hydroxymethylbenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With oxygen; palladium diacetate; sodium carbonate In cyclohexane at 130℃; for 48h; Molecular sieve; Schlenk technique; | 92% |
-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With 1,2-dibromo-1,1,2,2-tetrachloroethane; potassium carbonate; bis(4-methoxyphenyl)telluride In chloroform for 23h; Ambient temperature; | 90% |
| With 1,2-dibromo-1,1,2,2-tetrachloroethane; K2CO3 or triethylamine; bis(4-methoxyphenyl)telluride In chloroform for 23h; Ambient temperature; different ratios of halogenating agent and catalyst; different reaction time; different concentrations of aqueous base; | 90% |
| With benzeneseleninic anhydride In tetrahydrofuran for 24h; Ambient temperature; | 73% |

| Conditions | Yield |
|---|---|
| In dichloromethane at 40℃; for 5h; | 89% |

| Conditions | Yield |
|---|---|
| In dichloromethane at -78℃; for 0.5h; | 89% |

| Conditions | Yield |
|---|---|
| In pyridine | 89% |
| With silica gel HF-254/magnesium oxide at 20℃; for 4h; Green chemistry; chemoselective reaction; | 82% |
| With picoline In dichloromethane for 1h; Reflux; |

-
-
63971-78-8
tellurium dichloride

-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| In Petroleum ether | 88% |


-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| Stage #1: (3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-((R)-6-methylheptan-2-yl)-2,3,4,7,8,9,10,11,12,13,14,15,16,17-tetradecahydro-1H-cyclopenta [a]phenanthren-3-yl 4-bromobenzoate With palladium dichloride In water at 20℃; for 0.0333333h; Stage #2: With 1,1,3,3-Tetramethyldisiloxane for 11.9667h; | 86% |

-
-
63212-69-1
p-dimethylaminophenyl(p-methoxyphenyl)tellurium(II)

-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| 77% |


-
-
4456-34-2
bis(4-methoxyphenyl)telluride

-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| 72% |


| Conditions | Yield |
|---|---|
| With silica gel HF-254/magnesium oxide at 20℃; for 5.5h; Green chemistry; chemoselective reaction; | 72% |

| Conditions | Yield |
|---|---|
| With N,N,N,N,N,N-hexamethylphosphoric triamide; Bu3PI2 In diethyl ether for 6h; | 71% |
| With potassium hypophosphite; iodine; sodium sulfate In neat (no solvent) at 20℃; for 0.333333h; Milling; Green chemistry; | 53% |
-
-
57857-70-2
di-(p-methoxyphenyl)tellurium oxide

-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| 66% |

| Conditions | Yield |
|---|---|
| With triethylamine In N,N-dimethyl-formamide at 75 - 80℃; for 6h; | 62% |

-
-
34103-99-6
cholester-3β-yl thiobenzoate

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| In Petroleum ether | 58% |


| Conditions | Yield |
|---|---|
| With tetra-(n-butyl)ammonium iodide; triethylamine In N,N-dimethyl-formamide for 7h; Heating; | 43% |

| Conditions | Yield |
|---|---|
| With pyridine; ferric(III) bromide; oxygen In chlorobenzene at 130℃; Sealed tube; | 43% |
-
-
76303-24-7
cholest-5-en-3β-yl N-trifluoroacetylbenzimidate

-
A
-
604-32-0
cholesteryl benzoate

-
B
-
76303-25-8
α-ethoxy-α-trifluoroacetamidotoluene

| Conditions | Yield |
|---|---|
| With sodium hydrogen telluride In ethanol; dichloromethane for 2h; Ambient temperature; | A 13% B n/a |
-
-
57-88-5
cholesterol

-
-
99802-96-7
2-benzoyl-1,3-dimethyl-1H-imidazol-3-ium iodide

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With potassium carbonate In N,N-dimethyl-formamide at 80℃; for 14h; Inert atmosphere; Sealed tube; regioselective reaction; | 10% |

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid; toluene |
-
-
26048-46-4
7α-bromocholest-5-en-3β-ol benzoate

-
B
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With copper; acetic acid; zinc | |
| With nickel; ethyl acetate Hydrogenation.weiteres Reagens: Essigsaeure; | |
| With Lindlar's catalyst; ethyl acetate Hydrogenation.weiteres Reagens: Essigsaeure; |

| Conditions | Yield |
|---|---|
| With pyridine at 45 - 60℃; | |
| at 160℃; |

| Conditions | Yield |
|---|---|
| at 150 - 160℃; |

| Conditions | Yield |
|---|---|
| at 200℃; |
-
-
57-88-5
cholesterol

-
-
100936-11-6
2-methoxy-4-benzoyloxy-6-methyl pyrimidine

-
-
604-32-0
cholesteryl benzoate

| Conditions | Yield |
|---|---|
| With dmap Ambient temperature; Yield given; | |
| Ambient temperature; Yield given; |
-
-
67-66-3
chloroform

-
-
10034-85-2
hydrogen iodide

-
-
6557-19-3
5β,6β-epoxy-cholestan-3β-ol benzoate

-
-
604-32-0
cholesteryl benzoate



| Conditions | Yield |
|---|---|
| With dinitrogen monoxide; dioxo(tetramesitylporphyrinato)ruthenium(VI) In fluorobenzene at 100℃; under 7600 Torr; | 99% |
| With dinitrogen monoxide; dioxo(tetramesitylporphyrinato)ruthenium(VI) In fluorobenzene at 100℃; under 7500.6 Torr; for 3h; | 99% |
| With oxygen; trans-dioxo(5,10,15,20-tetramesitylporphirinato)ruthenium(VI) In benzene at 20℃; for 144h; | 93% |

| Conditions | Yield |
|---|---|
| With methanol; samarium(II) dibromide In tetrahydrofuran at 50℃; for 48h; Temperature; | 99% |

| Conditions | Yield |
|---|---|
| With tert.-butylhydroperoxide; bis(acetylacetonato) oxovanadium(IV) In benzene at 20℃; for 120h; Inert atmosphere; | 98% |
| With 3 A molecular sieve; pyridinium chlorochromate In benzene for 24h; Heating; | 89% |
| With tert.-butylhydroperoxide; [bis(acetoxy)iodo]benzene; magnesium acetate tetrahydrate In decane; butyl butyrate at 0℃; for 6h; regioselective reaction; | 78% |
-
-
604-32-0
cholesteryl benzoate

-
-
62307-95-3
3β-benzoyloxy-5α,6β-dichlorocholestane

| Conditions | Yield |
|---|---|
| With manganese(IV) oxide; acetic acid; acetyl chloride Ambient temperature; | 95% |
| With chloroform; antimony(III) chloride; chlorine at -20℃; | |
| With (Dichloroiodo)benzene In chloroform Heating; |
-
-
604-32-0
cholesteryl benzoate

-
-
109003-94-3, 116780-59-7
Benzoic acid (3S,8S,9S,10R,13R,14S,17R)-17-((R)-1,5-dimethyl-hexyl)-5-hydroxy-10,13-dimethyl-hexadecahydro-cyclopenta[a]phenanthren-3-yl ester

| Conditions | Yield |
|---|---|
| With oxygen; isopropyl alcohol; bis(trifluoroacetylacetonato)cobalt(II) at 75℃; for 8h; | 91% |
-
-
604-32-0
cholesteryl benzoate

-
A
-
6557-19-3
5β,6β-epoxy-cholestan-3β-ol benzoate

-
B
-
51646-05-0
5α,6α-epoxy-cholestan-3β-ol benzoate

| Conditions | Yield |
|---|---|
| With potassium permanganate; copper(II) sulfate In dichloromethane; water; tert-butyl alcohol for 2h; Yields of byproduct given; | A 90% B n/a |
| With monoperoxyphthalic acid; diethyl ether; chloroform | |
| With potassium permanganate; chloroform; acetic acid |

| Conditions | Yield |
|---|---|
| With magnesium(II) perchlorate; 9-ethyl-3,6-dimethyl-9H-carbazole In water; isopropyl alcohol at 23℃; for 2h; Irradiation; | 90% |
-
-
604-32-0
cholesteryl benzoate

-
-
250134-81-7
5,6-epoxycholestan-3β-ol benzoate

| Conditions | Yield |
|---|---|
| With perfluoro-cis-2-n-butyl-3-n-propyloxaziridine In various solvent(s) at -10℃; for 0.5h; | 81% |
-
-
604-32-0
cholesteryl benzoate

-
-
26048-46-4
7α-bromocholest-5-en-3β-ol benzoate

| Conditions | Yield |
|---|---|
| With 1,3-dibromo-5,5-dimethylimidazolidine-2,4-dione In hexane for 0.5h; Heating; | 74% |
| With N-Bromosuccinimide In tetrachloromethane for 0.5h; Heating; | 40% |
| With N-Bromosuccinimide; Petroleum ether |
-
-
604-32-0
cholesteryl benzoate

-
-
26048-47-5
5α,6β-dibromocholestan-3β-yl benzoate

| Conditions | Yield |
|---|---|
| With tetraethylammonium bromide In dichloromethane at 20℃; for 0.5h; Electrochemical reaction; | 72% |
-
-
614-45-9
tert-Butyl peroxybenzoate

-
-
604-32-0
cholesteryl benzoate

-
-
54758-42-8
7α-benzoyloxycholesteryl-3β-benzoate

| Conditions | Yield |
|---|---|
| Stage #1: cholesteryl benzoate With copper(I) bromide In dichloromethane for 0.25h; Heating; Stage #2: tert-Butyl peroxybenzoate In dichloromethane Heating; | 69% |
-
-
604-32-0
cholesteryl benzoate

-
-
50611-92-2
3β-benzoyloxycholest-5-ene-4-one

| Conditions | Yield |
|---|---|
| With perfluorooctylselenic acid; iodosylbenzene In various solvent(s) Heating; | 65% |
Cholesteryl benzoate Specification
The Cholesteryl benzoate with CAS registry number of 604-32-0 is also called Cholest-5-en-3-ol (3b)-,3-benzoate. The IUPAC name is[(3S,8S,9S,10R,13R,14S,17R)-10,13-dimethyl-17-[(2R)-6-methylheptan-2-yl]-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-yl] benzoate. Its EINECS registry number is 210-064-3. In addition, the molecular formula is C34H50O2 and the molecular weight is 490.76. It is a kind of white solid and belongs to the classes of Cholesteryl Compounds (Liquid Crystals); Functional Materials; Liquid Crystals; Related Compounds; Intermediates; Fine Chemicals; Pharmaceuticals; Steroids; Chiral Building Blocks; Complex Molecules.
Physical properties about this chemical are: (1)ACD/LogP: 12.68; (2)# of Rule of 5 Violations: 1; (3)ACD/LogD (pH 5.5): 12.68; (4)ACD/LogD (pH 7.4): 12.68; (5)ACD/BCF (pH 5.5): 1000000; (6)ACD/BCF (pH 7.4): 1000000; (7)ACD/KOC (pH 5.5): 10000000; (8)ACD/KOC (pH 7.4): 10000000; (9)#H bond acceptors: 2; (10)#H bond donors: 0; (11)#Freely Rotating Bonds: 8; (12)Polar Surface Area: 26.3 Å2; (13)Index of Refraction: 1.547; (14)Molar Refractivity: 149.64 cm3; (15)Molar Volume: 471.5 cm3; (16)Polarizability: 59.32×10-24cm3; (17)Surface Tension: 41.2 dyne/cm; (18)Density: 1.04 g/cm3; (19)Flash Point: 213.1 °C; (20)Enthalpy of Vaporization: 84.69 kJ/mol; (21)Boiling Point: 563.6 °C at 760 mmHg; (22)Vapour Pressure: 1E-12 mmHg at 25°C.
Preparation of Cholesteryl benzoate: it can be prepared by benzoic acid-[4]pyridyl ester and cholest-5-en-3b-ol. This reaction will need solvent CH2Cl2. The reaction time is 5 hours at reaction temperature of 40 °C. The yield is about 89%.
![Cholesteryl benzoate can be prepared by benzoic acid-[4]pyridyl ester and cholest-5-en-3b-ol](/UserFilesUpload/Preparation of Cholesteryl benzoate.png)
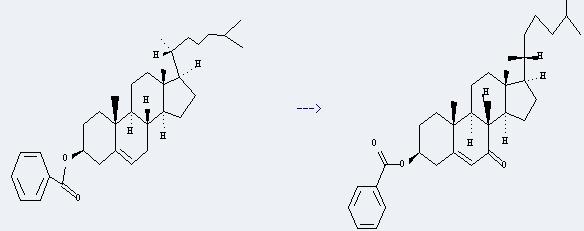
Uses of Cholesteryl benzoate: it can be used as intermediate of vitamin D3, and can be used in some hair colors, make-ups, and some other cosmetic preparations. Moreover, it can be used with cholesteryl nonanoate and cholesteryl oleyl carbonate in some thermochromic liquid crystals. In addition, it can be used to get 3b-benzoyloxy-cholest-5-en-7-one. This reaction will need reagent 3,5-dimethylpyrazolium fluorochromate and solvent acetonitrile. The reaction time is 10 hours by heating. The yield is about 70%.
When you are using this chemical, please be cautious about it as the following:
During using it, you should avoid contact with skin and eyes. In addition, you should not breathe dust.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(O[C@@H]4C/C3=C/C[C@@H]1[C@H](CC[C@]2([C@H]1CC[C@@H]2[C@H](C)CCCC(C)C)C)[C@@]3(C)CC4)c5ccccc5
(2)InChI: InChI=1/C34H50O2/c1-23(2)10-9-11-24(3)29-16-17-30-28-15-14-26-22-27(36-32(35)25-12-7-6-8-13-25)18-20-33(26,4)31(28)19-21-34(29,30)5/h6-8,12-14,23-24,27-31H,9-11,15-22H2,1-5H3/t24-,27+,28+,29-,30+,31+,33+,34-/m1/s1
(3)InChIKey: UVZUFUGNHDDLRQ-LLHZKFLPBM
Related Products
- Cholesteryl 4-nitrobenzoate
- Cholesteryl 4-nonylphenyl carbonate
- CHOLESTERYL ACETATE
- Cholesteryl behenate
- Cholesteryl benzoate
- Cholesteryl butyrate
- Cholesteryl caprylate
- Cholesteryl carbonate
- Cholesteryl chloride
- Cholesteryl chloroformate
- 604-33-1
- 60433-12-7
- 60434-13-1
- 60434-71-1
- 60434-95-9
- 604-35-3
- 60435-70-3
- 60439-16-9
- 60442-34-4
- 60442-41-3
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View