-
Name
Citral
- EINECS 226-394-6
- CAS No. 5392-40-5
- Article Data198
- CAS DataBase
- Density 0.856 g/cm3
- Solubility practically insoluble in water
- Melting Point < -10oC
- Formula C10H16O
- Boiling Point 229 °C at 760 mmHg
- Molecular Weight 152.236
- Flash Point 101.7 °C
- Transport Information
- Appearance yellow liquid
- Safety 24/25-37
- Risk Codes 38-43
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms 3,7-Dimethyl-2,6-octadien-1-al;3,7-Dimethyl-2,6-octadienal;Citral PQ Extra;Lemarome N;Lemsyn GB;NSC 6170;2,6-Octadienal,3,7-dimethyl-;
- PSA 17.07000
- LogP 2.87800
Citral Chemical Properties
Chemical Name: Citral
IUPAC NAME: (2E)-3,7-dimethylocta-2,6-dienal
CAS No.: 5392-40-5
EINECS: 226-394-6
RTECS: RG5075000
Molecular Formula: C10H16O
Molecular Weight: 152.23 g/mol
Melting Point: <-10°C
Density: 0.856 g/cm3
Flash Point: 101.7 °C
Boiling Point: 229 °C at 760 mmHg
3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5) is stable,but readily isomerizes and incompatible with alkalies, strong oxidizing agents, strong acids,and it is air and light sensitive.
Following is the structure of 3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5):
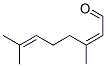
The chemical synonymous of 3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5) are (2E)-3,7-Dimethyl-2,6-octadienal ; 2,6-Octadienal, 3,7-dimethyl- ; 2,6-Octadienal,3,7-dimethyl- ; 3,7-Dimethyl-1,2,6-octadienal ; 3,7-Dimethyll-2,6-octadienal ; 3,7-dimethyl-octa-2,6-dienal ; 6-Octadienal,3,7-dimethyl-2 ; cis,trans-Citral
Citral Uses
3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5) is used as a perfumer agent for the preparation of lemon flavor, but also as raw materials for synthesis of ionone and vitamin A
Citral Toxicity Data With Reference
| 1. | skn-hmn 40 mg/24H MLD | FCTXAV Food and Cosmetics Toxicology. 17 (1979),259. | ||
| 2. | skn-man 16 mg/48H SEV | CTOIDG Cosmetics and Toiletries. 94 (8)(1979),41. | ||
| 3. | skn-rbt 100 mg/24H SEV | CTOIDG Cosmetics and Toiletries. 94 (8)(1979),41. | ||
| 4. | skn-gpg 1%/48H MOD | JSCCA5 Journal of the Society of Cosmetic Chemists. 28 (1977),357. | ||
| 5. | skn-gpg 100 mg/24H SEV | CTOIDG Cosmetics and Toiletries. 94 (8)(1979),41. | ||
| 6. | dnr-bcs 2222 µg/disc | OIGZSE Osaka-shi Igakkai Zasshi. Journal of Osaka City Medical Association. 34 (1985),267. | ||
| 7. | orl-rat LD50:4960 mg/kg | FCTXAV Food and Cosmetics Toxicology. 2 (1964),327. | ||
| 8. | ipr-rat LD50:460 mg/kg | JRPFA4 Journal of Reproduction and Fertility. 55 (1979),347. | ||
| 9. | orl-mus LD50:6000 mg/kg | BIJOAK Biochemical Journal. 34 (1940),1196. |
Citral Consensus Reports
Reported in EPA TSCA Inventory.
Citral Safety Profile
Moderately toxic by intraperitoneal route. Mildly toxic by ingestion. Experimental reproductive effects. A severe human and experimental skin irritant. Mutation data reported. Combustible liquid. When heated to decomposition it emits acrid smoke and irritating fumes.
Hazard Codes:
Xi: Harmful ![]()
Risk Statements about 3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5):
R38 Irritating to skin.
R43 May cause sensitization by skin contact.
Safety Statements about 3,7-DIMETHYL-2,6-OCTADIENAL (5392-40-5):
S24/25 Avoid contact with skin and eyes.
S37 Wear suitable gloves.
Citral Specification
1. Storage: Store in a cool, dry place. Store in a tightly closed container.
2. Handling: Do not breathe dust, vapor, mist, or gas. Do not get in eyes, on skin, or on clothing. Use only in a chemical fume hood.
3. Personal Protection: Eyes: Wear appropriate protective eyeglasses or chemical safety goggles as described by OSHA's eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166.Skin: Wear appropriate protective gloves to prevent skin exposure.Clothing: Wear appropriate protective clothing to prevent skin exposure.Respirators: Follow the OSHA respirator regulations found in 29 CFR 1910.134 or European Standard EN 149. Use a NIOSH/MSHA or European Standard EN 149 approved respirator if exposure limits are exceeded or if irritation or other symptoms are experienced.
4. Fire Fighting: As in any fire, wear a self-contained breathing apparatus in pressure-demand, MSHA/NIOSH (approved or equivalent), and full protective gear.Extinguishing Media: Use foam, dry chemical, or carbon dioxide.
5. Reactivity Profile Citral is an aldehyde. Aldehydes are frequently involved in self-condensation or polymerization reactions. These reactions are exothermic; they are often catalyzed by acid. Aldehydes are readily oxidized to give carboxylic acids. Flammable and/or toxic gases are generated by the combination of aldehydes with azo, diazo compounds, dithiocarbamates, nitrides, and strong reducing agents. Aldehydes can react with air to give first peroxo acids, and ultimately carboxylic acids. These autoxidation reactions are activated by light, catalyzed by salts of transition metals, and are autocatalytic (catalyzed by the products of the reaction). The addition of stabilizers (antioxidants) to shipments of aldehydes retards autoxidation. Citral can react with alkalis and strong acids. Citral can readily isomerize.
Related Products
- Citral
- CITRAL ETHYLENE GLYCOL ACETAL
- 53924-05-3
- 5392-67-6
- 5392-82-5
- 53928-30-6
- 5392-86-9
- 53929-59-2
- 53929-74-1
- 539-30-0
- 539-32-2
- 53933-06-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View