-
Name
Cobalt(II) oxide
- EINECS 215-154-6
- CAS No. 1307-96-6
- Article Data524
- CAS DataBase
- Density 6.45 g/cm3
- Solubility insoluble in water
- Melting Point 1785 °C
- Formula CoO
- Boiling Point 3800oC
- Molecular Weight 74.9926
- Flash Point
- Transport Information UN 3288 6.1/PG 3
- Appearance Black powder
- Safety 24-37-60-61
- Risk Codes 22-43-50/53
-
Molecular Structure
-
Hazard Symbols
 Xn,
Xn, N
N
- Synonyms C.I. 77322;C.I. Pigment Black 13;Cobalt Black;Cobalt monooxide;Cobalt monoxide;Cobalt(2+) oxide;Cobalt(II) oxide;Cobaltous oxide;Cobaltous oxide(CoO);FCO 178;Zaffre;
- PSA 17.07000
- LogP -0.11880
Synthetic route

| Conditions | Yield |
|---|---|
| In solid byproducts: LiCl; under N2; Li2O and metal halide ground together, added to glass ampoule, sealed under vacuum, sonicated (ultrasound) for 10 min, wrapped in glss wool, placed in oven at 450°C for 10 h; removed from oven, allowed to cool to room temp., trituration with THF for 10 h, ppt. and cloudy THF layer formed, evapn.of THF filtrate produced Li salt; or metal oxides washed with H2O for 10 min to remove Li salt; | 85% |
-
-
71-48-7, 917-69-1, 5931-89-5, 55881-15-7, 68931-68-0, 68931-69-1, 93029-27-7
cobalt(II) acetate

-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| In benzyl alcohol other Radiation; dispersed, microwave heating for 30-1200 s; XRD; | 60.8% |
| In solid byproducts: H2O, CO2, CO; Co(CH3CO2)2 was thermodestructed at 260°C; | |
| In neat (no solvent) N2 atmosphere; heating (300 - 400°C); detd. by powder X-ray diffraction; |
-
-
1277-43-6
cobaltocene

-
-
4358-26-3
tetraphenylborate anion

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

-
A
-
1307-96-6
cobalt(II) oxide

-
B
-
12184-35-9
(η5-cyclopentadienyl)-η4-cycloocta-1,5-dienecobalt(I)

-
E
-
7440-48-4
cobalt

| Conditions | Yield |
|---|---|
| With C5H5Na In tetrahydrofuran; water; pentane under N2, CoBr2*DME, NaCp, CoCp2 and COD mixed in THF in molar ratio of2/2.06/0.5/1.16 (CoCp2 added at -80°C prior to NaCp), warmed to room temp. during 3 h, evapd., pentane ext. passed through Al2O3/4% H2O and CuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 45.3% C n/a D n/a E n/a |
| With C5H5Na In tetrahydrofuran; water; pentane under N2, CoBr2*DME, NaCp, CoCp2 and COD mixed in THF in molar ratio of2/2.06/0.5/1.16 (CoCp2 added at -80°C after NaCp), warmed to room temp. during 3 h, evapd., pentane ext. passed through Al2O3/4% H2O andCuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 43% C n/a D n/a E n/a |
| With C5H5Na In tetrahydrofuran; water; pentane under N2, CoBr2*DME, NaCp, CoCp2 and COD mixed in THF in molar ratio of2/2.06/0.5/1.16 (CoCp2 added at 0°C prior to NaCp at -80°C), warmed to room temp. during 3 h, evapd., pentane ext. passed throughAl2O3/4% H2O and CuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 37.5% C n/a D n/a E n/a |
| With C5H5Na In tetrahydrofuran; water; pentane under N2, CoBr2*DME, NaCp, CoCp2 and COD mixed in THF in molar ratio of2/3/0.5/1.16, warmed to room temp. during 3 h, evapd., pentane ext. passed through Al2O3/4% H2O and CuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 11.5% C n/a D n/a E n/a |
| With C5H5Na In hexane; water; pentane under N2, CoBr2*DME, NaCp, CoCp2 and COD mixed in molar ratio of 2/3/0.5/1.16 in hexane at -80°C, warmed to room temp. during 3 h, evapd., pentane ext. passed through Al2O3/4% H2O and CuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 8.6% C n/a D n/a E n/a |

-
-
1277-43-6
cobaltocene

-
-
4358-26-3
tetraphenylborate anion

-
-
16733-97-4
cyclopentadienyllithium

-
-
5259-72-3, 10060-40-9, 111-78-4
1,5-dicyclooctadiene

-
A
-
1307-96-6
cobalt(II) oxide

-
B
-
12184-35-9
(η5-cyclopentadienyl)-η4-cycloocta-1,5-dienecobalt(I)

-
E
-
7440-48-4
cobalt

| Conditions | Yield |
|---|---|
| In tetrahydrofuran; water; pentane under N2, CoBr2*DME, LiCp, CoCp2 and COD mixed in THF in molar ratio of2/2.06/0.5/1.16, warmed to room temp. during 3 h, evapd., pentane ext. passed through Al2O3/4% H2O and CuCl powder; concd., cooled to -80°C, CpCoCOD filtered off; CuCl washed with water, CoCp2(1+) pptd. with BPh4(1-) from aq. soln., filtered off; pentane-insol. residue treated with water, Co and CoO mixt. filtered, Co(2+) determined complexometrically; | A n/a B 18% C n/a D n/a E n/a |


| Conditions | Yield |
|---|---|
| In neat (no solvent) 1700°C;; | 14.6% |
| In neat (no solvent) 1700°C;; | 14.6% |
| In neat (no solvent) byproducts: Co3O4; less than 500°C, 1Torr O2;; |

-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| at 20 - 800℃; | 13.5% |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2SO4; decompn.;; | |
| In neat (no solvent) byproducts: H2SO4; decompn.;; |

-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| With methane In neat (no solvent) reduction at 450°C;; | |
| In solid calcination (1050°C, N2 (low p(O2)) atmosphere); | |
| In neat (no solvent) heating to 1200°C in vacuum;; |

| Conditions | Yield |
|---|---|
| In solid byproducts: O2; heating Co2O3 to 1000°C under air; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; furnace heating (rate 4.5°C/min); gas chromy., X-ray diffraction; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) (Ar); In2O3 and Co reacted in 1:1 or 1:2 or 2:1 molar ratio; powdered inmortar; sealed under vacuum in quartz ampoule; heated to 723 K and with 0.5 K/h to 973 K; maintained for 7 days; cooled to 0.8 K/h to 473 K; le ft standing at room temp.; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; 300-400°C, p(H2O)=5-60 atm; drying (room temp.); X-ray diffraction; |

| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; 300°C, p(H2O)=5 atm; drying (room temp.); X-ray diffraction; |


| Conditions | Yield |
|---|---|
| at red heat;; | |
| equilibrium;; | |
| equilibrium;; |


| Conditions | Yield |
|---|---|
| In neat (no solvent) Kinetics; byproducts: O2; heating up to 600°C (in air); |


-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| In neat (no solvent) byproducts: H2O; multi stage react. on heating; sample placed in sample pan and heated gradually from 298 to 1200 K at 10 K/min under flowing air at rate of 100 mL/min; detn. by TG; | |
| In solid Co salt was heated at 823 K for 6 h; | |
| With CH3OCH2CH2OH In water mixt. stirred for 5 h; aged at room temp. for 8 h; film deposited by spin coating on SiO2 substrate; XRD; |

-
A
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| In not given a soln. containing 2 moles Co(NO3)2*6H2O and 1 mole Mn(NO3)2*6H2O is evaporated and the residue heated at 850 °C;; product mixture obtained;; |

-
-
24849-27-2
Co(N-(6-methyl-2-pyridyl)salicylaldimine)2

-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| With sodium hydroxide In water 60°C; |

| Conditions | Yield |
|---|---|
| In solid 354+/-3°C; | |
| In neat (no solvent, solid phase) 354+/-3°C; |


| Conditions | Yield |
|---|---|
| In solid 356+/-1°C; | |
| In neat (no solvent, solid phase) 356+/-1°C; |


| Conditions | Yield |
|---|---|
| In melt Electrolysis; (N2); at 650-700.degreeC; voltages 3-5 V; current 1 A; 99.7 mol. % PO3(1-) in the melt; graphite anode and cathode or platinum anode and silver cathode; elem. anal.; | 100% |

| Conditions | Yield |
|---|---|
| In melt Electrolysis; (N2); at 650-700.degreeC; voltages 3-5 V; current 1 A; 97.6 mol. % PO3(1-) in the melt; graphite anode and cathode or platinum anode and silver cathode; elem. anal.; | A 95% B 5% |


| Conditions | Yield |
|---|---|
| In melt Electrolysis; (N2); at 650-700°C; voltages 3-5 V; current 1 A; graphite electrodes; 95 mol.% metaarsenite in the melt; | A 10% B 90% |


| Conditions | Yield |
|---|---|
| In melt Electrolysis; (N2); at 650-700.degreeC; voltages 3-5 V; current 1 A; 99.3 mol. % PO3(1-) in the melt; graphite anode and cathode or platinum anode and silver cathode; elem. anal.; | A 88% B 12% |
| In melt Electrolysis; (N2); at 650-700.degreeC; voltages 3-5 V; current 1 A; 99.0 mol. % PO3(1-) in the melt; graphite anode and cathode or platinum anode and silver cathode; elem. anal.; | A 50% B 50% |


| Conditions | Yield |
|---|---|
| With O2 In further solvent(s) dione as solvent, reflux for 24 h under O2; cooling to room temp., evapn. in vac., extn. with acetone, evapn. at room temp. in air or in vac., sublimation, recrystn. from EtOH, elem. anal.; | 85% |

| Conditions | Yield |
|---|---|
| at 350℃; for 72h; Sealed tube; | 82.35% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixt. of MoO3, CoO suspended in H2O; pH value of soln. before hydrothermal treatment was 4.1; mixt. put into Teflon-lined autoclave and heated in a forced convection oven at 453 K under autogenous pressure for 1 ds or heated at 423 K; filtered; washed (H2O); dried in air at room temp.; monitored by X-ray diffraction; | 81.6% |

| Conditions | Yield |
|---|---|
| In water High Pressure; mixt. of MoO3, CoO suspended in H2O; pH value of soln. before hydrothermal treatment was 4.1; mixt. put into Teflon-lined autoclave and heated in a forced convection oven at 453 K under autogenous pressure for 3 ds; filtered; washed (H2O); dried in air at room temp.; monitored by X-ray diffraction; elem. anal.; | 73.8% |
-
-
1307-96-6
cobalt(II) oxide

-
-
10397-52-1
3,3’,5,5’-tetracarboxydiphenylmethane

-
-
7732-18-5
water

-
-
68-12-2, 33513-42-7
N,N-dimethyl-formamide

| Conditions | Yield |
|---|---|
| In water; N,N-dimethyl-formamide High Pressure; mixt. of CoO, acid, DMF and H2O was heated in reactor at 105°C for 3 d; cooled to room temp. at rate 10°C/h; elem. anal.; | 72% |

-
-
1307-96-6
cobalt(II) oxide

-
-
86119-84-8, 7664-38-2
phosphoric acid

-
-
6153-56-6
oxalic acid dihydrate

-
-
110-60-1
1,4-diaminobutane

| Conditions | Yield |
|---|---|
| In water at 170℃; for 168h; Autoclave; | 70.2% |


| Conditions | Yield |
|---|---|
| With Na2CO3 In melt Electrolysis; (N2); at 650-700°C; voltages 3-5 V; current 1 A; graphite electrodes; 95 mol.% metaantimonate in the melt; | A 70% B 29% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Tb compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 70% |


| Conditions | Yield |
|---|---|
| With hydrogenchloride In water at 230℃; for 72h; Autoclave; | 68% |


| Conditions | Yield |
|---|---|
| In neat (no solvent) at 160℃; Autoclave; | 67.2% |
-
-
1307-96-6
cobalt(II) oxide

| Conditions | Yield |
|---|---|
| In water under N2; addn. of CoO to Ir compd. in water, stirred for 10 min at 80°C, then addn. of CoBr2, stirring at 80°C for 4 h; filtered, filtrate allowed to stand at room temp. for 2 d, crystn., collected by filtration; elem. anal.; | 66% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Eu compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 65% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Pr compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 63% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Yb compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 62% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Sm compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 60% |


| Conditions | Yield |
|---|---|
| In water High Pressure; mixing C5H3N(CO2H)2, CoO, Dy compd., H2O in steel vessel, heating to 180°C for 3 d, cooling to room temp. (1.5°C/h); isolation of crystals, washing with H2O and Et2O, elem. anal.; | 60% |


-
-
1307-96-6
cobalt(II) oxide

-
-
7681-65-4
copper(l) iodide

-
-
10034-85-2
hydrogen iodide

-
-
7681-11-0
potassium iodide

| Conditions | Yield |
|---|---|
| In methanol at 20 - 145℃; for 168h; Autoclave; | 60% |


| Conditions | Yield |
|---|---|
| In water at 580℃; for 168h; Sealed tube; Autoclave; | A n/a B 50% |

-
-
1307-96-6
cobalt(II) oxide

-
-
77-92-9
citric acid

-
-
1278403-62-5
[(zinc(II))(cobalt(II))(citric acid(-3H))(bromide)]

| Conditions | Yield |
|---|---|
| In melt High Pressure; mixt. of CoO (0.680 mmol), ZnBr2 (7.370 mmol) and citric acid (31.302 mmol) heated in Teflon-lined stainless steel reactor at 170°C for 6d, slowly cooled to room temp.; ppt. sepd., washed with H2O and EtOH, dried in air; elem. anal.; | 40.25% |


| Conditions | Yield |
|---|---|
| at 580℃; under 1500150 Torr; Autoclave; | 40% |

-
-
1307-96-6
cobalt(II) oxide

-
-
77-92-9
citric acid

-
-
7646-85-7
zinc(II) chloride

-
-
1278403-61-4
[(zinc(II))(cobalt(II))(citric acid(-3H))(chloride)]

| Conditions | Yield |
|---|---|
| In melt High Pressure; mixt. of CoO (0.680 mmol), ZnCl2 (7.330 mmol) and citric acid (31.302 mmol) heated in Teflon-lined stainless steel reactor at 170°C for 6d, slowly cooled to room temp.; ppt. sepd., washed with H2O and EtOH, dried in air; elem. anal.; | 36.29% |

Cobalt oxide Chemical Properties
Chemistry informtion about Cobalt(II) Oxide (CAS NO.1307-96-6) is:
IUPAC Name: Oxocobalt
Synonyms: C.I. 77322 ; C.I. Pigment Black 13 ; C.I.77322 ; C.I.pigmentblack13 ; CI77322 ; CIpigmentblack13 ; Cobalt monooxide ; Cobalt Oxide (CoO)
Product Categories: Inorganic & Organic Chemicals ; Inorganics
MF: CoO
MW: 74.93
EINECS: 215-154-6
Density: 6.45 g/cm3
Melting Point: 1785 °C
Water Solubility: insoluble
Sensitive: Air Sensitive
Merck: 14,2446
Stability: Stability Stable, but may be moisture sensitive.
Following is the molecular structure of Cobalt(II) Oxide (CAS NO.1307-96-6) is:
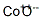
Cobalt oxide Uses
Cobalt(II) Oxide (CAS NO.1307-96-6) is used in pigments for ceramics, glass coloring & decolorization, prepn of cobalt metal catalysts, oxidation catalyst for drying oils, fast drying paints & varnishes, cobalt powder for binder in sintered tungsten carbide, in semiconductors.It alsohas for centuries used as a coloring agent on kiln fired pottery, the earliest examples go back to 12th century German pottery.[6] The additive provides a deep shade of blue named Cobalt blue. The band gap (CoO) is around 2.4 eV.
Cobalt oxide Production
Raw Materials are Sodium Hydroxide-->Sulfuric Acid -->Nitric Acid-->Sodium Carbonate-->Ammonium Hydroxide-->Sodium Hypochlorite-->Oxalic Acid-->Cobalt Chloride-->Extractant-->Chloric Acid-->Cobalt .
Preparation Products are Ammonia Synthesis Catalyst-->Porcelain Glaze,Industrial-->Ceramic Pigment.
Cobalt oxide Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| dog | LDLo | oral | 89mg/kg (89mg/kg) | Environmental Quality and Safety, Supplement. Vol. 1, Pg. 1, 1975. | |
| mouse | LD50 | subcutaneous | 125mg/kg (125mg/kg) | Zhurnal Vsesoyuznogo Khimicheskogo Obshchestva im. D.I. Mendeleeva. Journal of the D.I. Mendeleeva All-Union Chemical Society. Vol. 19, Pg. 186, 1974. | |
| mouse | LDLo | intramuscular | 400mg/kg (400mg/kg) | Cancer Research. Vol. 22, Pg. 152, 1962. | |
| rat | LD50 | oral | 202mg/kg (202mg/kg) | behavioral: somnolence (general depressed activity) gastrointestinal: "hypermotility, diarrhea" | Food and Chemical Toxicology. Vol. 20, Pg. 311, 1982. |
| rat | LDLo | intratracheal | 50mg/kg (50mg/kg) | National Technical Information Service. Vol. AEC-TR-6710, |
Cobalt oxide Consensus Reports
IARC Cancer Review: Group 2B IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Animal Sufficient Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) ; Human Inadequate Evidence IMEMDT IARC Monographs on the Evaluation of Carcinogenic Risk of Chemicals to Man . 52 , 1991,p. 363.(World Health Organization, Internation Agency for Research on Cancer,Lyon, France.: ) (Single copies can be ordered from WHO Publications Centre U.S.A., 49 Sheridan Avenue, Albany, NY 12210) . Cobalt and its compounds are on the Community Right-To-Know List. Reported in EPA TSCA Inventory.
Cobalt oxide Safety Profile
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Poison by ingestion, subcutaneous, and intratracheal routes. Moderately toxic by intramuscular route. Violent reaction with hydrogen peroxide. See also COBALT. Note: The commercial oxides are usually not definite chemical compounds but mixtures of the cobalt oxides.
Hazard Codes:
 Xn
Xn
 N
N
Risk Statements:
R22:Harmful if swallowed.
R43:May cause sensitization by skin contact.
R50/53:Very toxic to aquatic organisms, may cause long-term adverse effects in the aquatic environment.
Safety Statements:
S24:Avoid contact with skin.
S37:Wear suitable gloves.
S60:This material and its container must be disposed of as hazardous waste.
S61:Avoid release to the environment. Refer to special instructions / safety data sheets.
RIDADR: UN 3288 6.1/PG 3
WGK Germany: 3
RTECS: GG2800000
HazardClass: 6.1(b)
PackingGroup: III
Cobalt oxide Analytical Methods
For occupational chemical analysis use NIOSH: Cobalt, 7027; Elements, 7300.
Cobalt oxide Specification
Cobalt(II) Oxide (CAS NO.1307-96-6) is a olive green, red, gray or black, odorless powder.
First Aid Measures:
Ingestion: Never give anything by mouth to an unconscious person. Get medical aid. Do not induce vomiting. If conscious and alert, rinse mouth and drink 2-4 cupfuls of milk or water. Seek medical attention. If individual is drowsy or unconscious, do not give anything by mouth; place individual on the left side with the head down. Contact a physician, medical facility, or poison control center for advice about whether to induce vomiting. If possible, do not leave individual unattended.
Inhalation: Remove from exposure to fresh air immediately. If not breathing, give artificial respiration. If breathing is difficult, give oxygen. Get medical aid. If symptoms develop, move individual away from exposure and into fresh air. If symptoms persist, seek medical attention. If breathing is difficult, administer oxygen. Keep person warm and quiet; seek immediate medical attention. May cause respiratory tract irritation. The toxicological properties of this substance have not been fully investigated.
Skin: May cause skin irritation.Get medical aid. Flush skin with plenty of soap and water for at least 15 minutes while removing contaminated clothing and shoes. Wash clothing before reuse. Remove contaminated clothing and wash exposed area thoroughly with soap and water. A physician should examine the area if irritation or pain persists.
Eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Get medical aid.May cause eye irritation. Irrigate exposed eyes with copious amounts of tepid water for at least 15 minutes. If irritation, pain, swelling, lacrimation, or photophobia persist, the patient should be seen in a health care facility.
Handling and Storage:
Storage: Kepp container tightly closed. Suitable for any general chemical storage area. Store in a tightly closed container. Store in a cool, dry, well-ventilated area away from incompatible substances.
Handling: Wash thoroughly after handling. Remove contaminated clothing and wash before reuse. Use with adequate ventilation. Minimize dust generation and accumulation. Avoid contact with eyes, skin, and clothing. Keep container tightly closed. Avoid ingestion and inhalation. All chemicals should be considered hazardous. Avoid direct physical contact. Use appropriate, approved safety equipment. Untrained individuals should not handle this chemical or its container. Handling should occur in a chemical fume hood.
Related Products
- Cobalt
- Cobalt acetate
- Cobalt alloy
- COBALT ALLOY, Co,Cr
- Cobalt bis(2-ethylhexanoate)
- Cobalt bromide (CoBr<sub>2</sub>)
- COBALT HYDROCARBONYL
- Cobalt hydroxide
- Cobalt hydroxide oxide
- Cobalt iodide,dihydrate (8CI,9CI)
- 130798-48-0
- 130800-90-7
- 13080-43-8
- 1308-04-9
- 1308-06-1
- 13080-85-8
- 13080-86-9
- 13080-89-2
- 13080-90-5
- 13081-14-6
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View