-
Name
Cycloheptanone
- EINECS 207-937-6
- CAS No. 502-42-1
- Article Data367
- CAS DataBase
- Density 0.929 g/cm3
- Solubility Insoluble in water
- Melting Point -21 °C
- Formula C7H12O
- Boiling Point 180 °C at 760 mmHg
- Molecular Weight 112.172
- Flash Point 55.6 °C
- Transport Information UN 1987 3/PG 3
- Appearance clear colorless to yellow liquid
- Safety 23-24/25-36-26
- Risk Codes 10-36/37/38
-
Molecular Structure
-
Hazard Symbols
 Xi
Xi
- Synonyms Ketocycloheptane;Ketoheptamethylene;NSC 9471;Suberon;Suberone;
- PSA 17.07000
- LogP 1.90970
Synthetic route

| Conditions | Yield |
|---|---|
| With ruthenium trichloride; iodobenzene; potassium peroxomonosulfate In water; acetonitrile at 20℃; for 0.8h; | 100% |
| With ruthenium trichloride; iodobenzene; potassium peroxymonosulfate In water; acetonitrile at 20℃; for 0.8h; Inert atmosphere; | 100% |
| With 5 wt% Pd nanoparticles loaded on phosphate anion exchanged [Mg6Al2(OH)16]CO3*xH2O; air at 50℃; under 760.051 Torr; for 6h; Reagent/catalyst; Irradiation; | 100% |
-
-
36237-80-6
cycloheptanone semicarbazone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 3h; Heating; | 100% |
-
-
77378-96-2
1-cycloheptylidenehydrazine

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 2h; Heating; | 100% |
| With HOF* CH3CN In dichloromethane at 0℃; for 0.0166667h; |

| Conditions | Yield |
|---|---|
| With hydrogen; palladium(II) complex of ferrocenylamine sulfide (3) In acetone under 4137.2 Torr; for 8h; | 95% |
| With hydrogen In ethanol at 20℃; under 760.051 Torr; for 1h; chemoselective reaction; | 94% |
| With hydrogen; palladium |

| Conditions | Yield |
|---|---|
| With Oxone; water; potassium hydrogencarbonate In acetone for 0.25h; Heating; | 95% |
| With Cu(NO3)2-SiO2 for 0.166667h; oxime cleavage; microwave irradiation; | 95% |
| With γ-picolinium chlorochromate; silica gel In dichloromethane at 20℃; for 4h; | 93% |
-
-
25632-02-4
1,1-dimethoxycycloheptane

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With Methyltrichlorosilane; sodium iodide In acetonitrile for 0.5h; Ambient temperature; | 95% |
-
-
1056246-49-1
2-bromocycloheptanone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With hydrogen iodide for 0.0833333h; Ambient temperature; | 93% |
| With chloro-trimethyl-silane; sodium iodide In acetonitrile for 8h; Ambient temperature; | 88% |
-
-
64-18-6
formic acid

-
-
3349-69-7
1-cycloheptylidene-2-phenylhydrazine

-
A
-
622-84-4
2-formyl-1-phenylhydrazine

-
B
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| In ethanol for 5h; Heating; | A 93% B 87% |

-
-
3349-69-7
1-cycloheptylidene-2-phenylhydrazine

-
A
-
622-84-4
2-formyl-1-phenylhydrazine

-
B
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With formic acid In ethanol for 5h; Heating; | A 93% B 87% |
-
-
72170-88-8
Cycloheptanone SAMP-hydrazone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With selenium(IV) oxide; dihydrogen peroxide In methanol; water at 20℃; pH=7; aq. phosphate buffer; | 93% |

| Conditions | Yield |
|---|---|
| With trimethylsilyl trifluoromethanesulfonate; nitrobenzaldehyde polymer In dichloromethane for 3h; Ambient temperature; | 92% |
| With 4-nitrobenzaldehdye; trimethylsilyl trifluoromethanesulfonate In dichloromethane for 0.0833333h; Ambient temperature; | 78% |
-
-
6669-45-0, 88765-15-5, 88765-17-7
1,2-epoxy-3-cycloheptene

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With BF4; hydrogen In dichloromethane under 10343 Torr; for 5h; Ambient temperature; | 91% |

| Conditions | Yield |
|---|---|
| With potassium permanganate; copper(II) sulfate In dichloromethane for 24h; Ambient temperature; | 90% |
| With 2,2,6,6-Tetramethyl-1-piperidinyloxy free radical; [bis(acetoxy)iodo]benzene In dichloromethane at 0 - 20℃; for 0.333333h; Inert atmosphere; Green chemistry; | 85% |
| With iodosylbenzene In water at 0℃; for 1h; | 52% |

| Conditions | Yield |
|---|---|
| With ammonium cerium(IV) nitrate In methanol; water at 20℃; for 0.166667h; Ring cleavage; | 90% |
| With chloral hydrate In hexane at 25℃; for 0.5h; Inert atmosphere; | 84% |
| With tellurium; sodium tetrahydroborate; water 1.) EtOH, 25 deg C, 30 min; Multistep reaction; |

| Conditions | Yield |
|---|---|
| With Celite; pyridinium chlorochromate In dichloromethane for 30h; Heating; highly selective oxidative cleavage; | A 90% B 87% |
-
-
56382-69-5
cycloheptanone p-tolylsulfonylhydrazone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| Amberlyst 15 In water; acetone for 23h; Ambient temperature; | 90% |
| With copper(II) sulfate In tetrahydrofuran; methanol; water for 4h; Heating; | 85% |

| Conditions | Yield |
|---|---|
| indium(III) chloride In methanol; water for 1.33333h; Heating; | 90% |
-
-
61612-52-0
Cycloheptyloxy-trimethyl-silane

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen; cobalt(II) benzoate In acetonitrile at 20℃; for 10h; | 89% |
| With sodium bromate; ammonium chloride In water; acetonitrile at 80℃; for 0.833333h; | 81% |

| Conditions | Yield |
|---|---|
| With fluorosulfonylchloride In diethyl ether; water Ambient temperature; | 85% |
| With sodium nitrate; sulfuric acid; silica gel In dichloromethane at 20℃; for 0.416667h; | 85% |
-
-
75778-52-8
nitrosyl(5,10,15,20-tetraphenylporphyrinato)(pyridine)cobalt(III)

-
-
109-63-7
boron trifluoride diethyl etherate

-
B
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With cycloheptanol In 1,2-dichloro-ethane byproducts: BF3-pyridine complex, H2O; under Ar addn. of BF3*Et2O to mixt. of alcohol and complex in (CH2)2Cl2, further addn. of BF3*Et2O, heating in 60°C oil bath for 45 min,addn. 1 drop of pyridine in hexane, removing BF3-pyridine complex pptd.; solvent removing in vac., addn. of petroleum ether to resulting oil, filtration, drying under Ar; | A n/a B 84% |
| With Cyclopentanol In 1,2-dichloro-ethane byproducts: BF3-pyridine complex, H2O; under Ar addn. of BF3*Et2O to mixt. of alcohol and complex in (CH2)2Cl2, further addn. of BF3*Et2O, heating in 60°C oil bath for 30 min,addn. 1 drop of pyridine in hexane, removing BF3 complex pptd.; solvent removing in vac., addn. of petroleum ether to resulting oil, filtration, drying under Ar; | A n/a B 54% |


| Conditions | Yield |
|---|---|
| With cycloheptanol In 1,2-dichloro-ethane byproducts: BF3-pyridine complex, H2O; under Ar addn. of BF3*Et2O to mixt. of alcohol and complex in (CH2)2Cl2, further addn. of BF3*Et2O, heating in 60°C oil bath for 10 min,addn 1 drop pyridine, filtration, further conditions: various concn. ofLewis acid, time, various yields; solvent removing in vac., addn. of petroleum ether to resulting oil, filtration, drying under Ar; | A n/a B 84% |


| Conditions | Yield |
|---|---|
| With dihydrogen peroxide In water; acetonitrile at 55℃; for 12h; Wacker Oxidation; | 83% |
| With oxygen; palladium(II) sulfate; PdSO4-H3PMo6W6O40 In cyclohexane; water at 30℃; for 22h; | 10% |
| With oxygen; H3PMo6W6O40 In cyclohexane; water at 29.9℃; under 760 Torr; for 22h; | 10% |
-
-
75031-88-8
Cycloheptanthion

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With nitrosonium tetrafluoroborate In dichloromethane for 0.5h; Ambient temperature; | 83% |
-
-
39672-01-0
cycloheptanone dimethylhydrazone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With ferric nitrate In dichloromethane 30 min., r.t., then reflux; | 82% |
| tin(ll) chloride; palladium dichloride In water for 0.025h; microwave irradiation; | 82% |
| With montmorillonite K-10 for 0.0333333h; microwave irradiation; | 88 % Chromat. |

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With N-hydroxyphthalimide; oxygen; cobalt(II) benzoate In acetonitrile at 20℃; for 12h; | 82% |

| Conditions | Yield |
|---|---|
| With oxygen; trifluoroacetic acid; sodium nitrite at 0 - 20℃; for 12.25h; Product distribution / selectivity; | A 80% B 18% |
| With oxygen; sodium nitrite In trifluoroacetic acid at 0 - 20℃; for 12h; | A 73% B 18% |
-
-
354988-81-1
cycloheptanone thiosemicarbazone

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With nickel(II) perchlorate In ethanol for 5h; Heating; | 76.36% |
-
-
72946-37-3
1-hydroxy-1-(2-phenylethynyl)cycloheptane

-
-
156642-47-6
1-iodo-2-((2-methylallyl)oxy)benzene

-
A
-
1603105-46-9
3-methyl-3-(3-phenylprop-2-yn-1-yl)-2,3-dihydrobenzofuran

-
B
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With tris-(dibenzylideneacetone)dipalladium(0); tetrabutylammomium bromide; potassium carbonate In water at 100℃; Microwave irradiation; Inert atmosphere; Sealed tube; Green chemistry; | A 76% B n/a |


| Conditions | Yield |
|---|---|
| With boron trifluoride diethyl etherate; tetra-(n-butyl)ammonium iodide In dichloromethane at 25℃; for 4h; | A 72% B n/a |

| Conditions | Yield |
|---|---|
| 70% | |
| In not given IR, PMR, mass spectral data, GLC;; | 70% |

| Conditions | Yield |
|---|---|
| With 3-chloro-benzenecarboperoxoic acid In dichloromethane at 20℃; for 120h; Baeyer-Villiger reaction; | 100% |
| With oxygen; 1-(n-butyl)-3-methylimidazolium triflate at 20℃; for 0.25h; Baeyer-Villiger Ketone Oxidation; Electrochemical reaction; Green chemistry; | 97% |
| With Ag/WO3 nanobars; dihydrogen peroxide In acetonitrile at 80℃; for 9h; Baeyer-Villiger Ketone Oxidation; Green chemistry; | 96% |

| Conditions | Yield |
|---|---|
| With hydroxylamine hydrochloride; sodium acetate In methanol Heating; | 100% |
| With pyridine; hydroxylamine hydrochloride In ethanol at 90 - 100℃; for 2h; | 100% |
| With hydroxylamine hydrochloride; sodium acetate In diethyl ether at 20℃; for 9h; | 98% |

| Conditions | Yield |
|---|---|
| With aluminum oxide; sodium tetrahydroborate In hexane at 20℃; for 3h; | 100% |
| With C15H18BF3; hydrogen; tert-butylimino-tri(pyrrolidino)phosphorane In tetrahydrofuran at 75℃; under 75007.5 Torr; for 20h; Glovebox; | 99% |
| With hydrogen; silver perchlorate; potassium hexamethylsilazane In toluene at 25℃; under 15001.5 Torr; for 17h; Glovebox; | 99% |
-
-
502-42-1
cycloheptanone

-
-
1056246-49-1
2-bromocycloheptanone

| Conditions | Yield |
|---|---|
| With N-Bromosuccinimide; toluene-4-sulfonic acid at 20℃; for 2.5h; | 100% |
| With N-Bromosuccinimide; toluene-4-sulfonic acid In dichloromethane for 16h; Cooling with ice; | 99% |
| With N-Bromosuccinimide In dimethyl sulfoxide at 20 - 65℃; | 95% |

| Conditions | Yield |
|---|---|
| In diethyl ether at -78℃; | 100% |

| Conditions | Yield |
|---|---|
| Nafion-H In benzene Heating; | 100% |
| With perchloric acid; silica gel at 25 - 30℃; for 0.05h; | 97% |
| With P-benzyltriphenylphosphonium tribromide at 20℃; for 1.5h; | 96% |

| Conditions | Yield |
|---|---|
| With trichloroacetic acid at 100℃; for 0.0833333h; Fischer indole synthesis; | 100% |
| With 1,3-bis(3-sulfopropyl)-1H-imidazol-3-ium hydrogensulfate In water at 80℃; for 1h; Fischer indole synthesis; | 88% |
| With acetic acid for 0.5h; Heating; | 74% |
-
-
1576-35-8
toluene-4-sulfonic acid hydrazide

-
-
502-42-1
cycloheptanone

-
-
56382-69-5
cycloheptanone p-tolylsulfonylhydrazone

| Conditions | Yield |
|---|---|
| In methanol at 20℃; Schlenk technique; | 100% |
| In ethanol at 100℃; for 0.25h; Inert atmosphere; | 99% |
| In ethanol for 2h; Reflux; Inert atmosphere; | 90% |
-
-
616-38-6
carbonic acid dimethyl ester

-
-
502-42-1
cycloheptanone

-
-
52784-32-4
2-(methoxycarbonyl)cycloheptanone

| Conditions | Yield |
|---|---|
| Stage #1: carbonic acid dimethyl ester With sodium hydride In toluene; mineral oil for 1h; Reflux; Stage #2: cycloheptanone In toluene; mineral oil for 3h; Reflux; | 100% |
| Stage #1: carbonic acid dimethyl ester; cycloheptanone With sodium hydride In mineral oil; benzene for 3.84h; Inert atmosphere; Reflux; Stage #2: With acetic acid In mineral oil; benzene at 0℃; Inert atmosphere; | 94% |
| With sodium hydride In benzene for 3h; Reflux; | 94% |

| Conditions | Yield |
|---|---|
| In benzene for 7h; Heating; | 100% |
-
-
69739-34-0
t-butyldimethylsiyl triflate

-
-
502-42-1
cycloheptanone

-
-
68081-19-6
tert-butyl (cyclohept-1-en-1-yloxy)dimethylsilane

| Conditions | Yield |
|---|---|
| With triethylamine In dichloromethane for 0.0833333h; | 100% |
| With triethylamine In dichloromethane at 0℃; | 59% |
| With triethylamine In dichloromethane at 0℃; for 0.666667h; |

| Conditions | Yield |
|---|---|
| In tetrahydrofuran at 0 - 23℃; for 18h; Inert atmosphere; | 100% |
| In tetrahydrofuran; diethyl ether at -5 - 20℃; | 86% |
| In tetrahydrofuran at -78℃; Grignard reaction; | 66% |
-
-
221636-54-0
5,7-bis(trifluoroacetyl)-8-quinolylamine

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With ammonia; water In acetonitrile at 20℃; for 48h; Condensation; Cyclization; | 100% |
-
-
75-52-5
nitromethane

-
-
100-53-8
phenylmethanethiol

-
-
502-42-1
cycloheptanone

-
-
335458-25-8
1-benzylthio-1-nitromethylcycloheptane

| Conditions | Yield |
|---|---|
| With piperidine In benzene | 100% |
| With ethylenediamine In acetonitrile for 12h; Heating; | 83% |


| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid at 100℃; for 24h; Heating; | 100% |
-
-
16280-66-3
5-phenyl-3-(2′-pyridyl)-1,2,4-triazine

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With 4 Angstroem molecular sives; N-methyl-ethane-1,2-diamine In toluene for 22h; Diels-Alder reaction; Heating; | 100% |
| With 4 A molecular sieve; N-methyl-ethane-1,2-diamine In toluene at 120℃; for 22h; | 100% |

| Conditions | Yield |
|---|---|
| With trimethyl orthoformate at 60℃; under 6000480 Torr; for 16h; | 100% |
| With trimethyl orthoformate at 20℃; for 1h; | 92% |
| With Mo-TiO2/rGO at 20℃; for 0.5h; Catalytic behavior; |
-
-
23304-24-7
p-tolyldiazomethane

-
-
502-42-1
cycloheptanone

-
-
1278594-26-5
(S)-2-(4-methylphenyl)cyclooctanone

| Conditions | Yield |
|---|---|
| Stage #1: cycloheptanone With C33H29N3O3; scandium tris(trifluoromethanesulfonate) In toluene for 0.25h; Inert atmosphere; Stage #2: p-tolyldiazomethane In toluene at -78℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 100% |
| Stage #1: cycloheptanone With 1,1,1-tris[(4R,5S)-4,5-indanediyloxazolin-2-yl]ethane; scandium tris(trifluoromethanesulfonate) In toluene for 0.25h; Inert atmosphere; Stage #2: p-tolyldiazomethane In toluene at -78℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 98% |
-
-
65864-99-5
1-(diazomethyl)-3-methoxybenzene

-
-
502-42-1
cycloheptanone

-
-
1278594-27-6
(S)-2-(3-methoxyphenyl)cyclooctanone

| Conditions | Yield |
|---|---|
| Stage #1: cycloheptanone With C33H29N3O3; scandium tris(trifluoromethanesulfonate) In toluene for 0.25h; Inert atmosphere; Stage #2: 1-(diazomethyl)-3-methoxybenzene In toluene at -78℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 100% |
| Stage #1: cycloheptanone With 1,1,1-tris[(4R,5S)-4,5-indanediyloxazolin-2-yl]ethane; scandium tris(trifluoromethanesulfonate) In toluene for 0.25h; Inert atmosphere; Stage #2: 1-(diazomethyl)-3-methoxybenzene In toluene at -78℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 98% |

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With hydrogenchloride; palladium 10% on activated carbon; hydrogen In methanol; water at 20℃; for 13h; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 1.4-dibromobenzene With n-butyllithium In tetrahydrofuran at -75 - -70℃; Inert atmosphere; Stage #2: cycloheptanone In tetrahydrofuran at -40℃; Inert atmosphere; | 100% |
| Stage #1: 1.4-dibromobenzene With iodine; magnesium In diethyl ether Grignard Reaction; Reflux; Stage #2: cycloheptanone In diethyl ether at 20℃; for 2h; |

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| With ammonium hydroxide In acetonitrile at 50℃; for 96h; | 100% |
-
-
16182-15-3
2,4,6-trimethylbenzenesulfonohydrazide

-
-
502-42-1
cycloheptanone

| Conditions | Yield |
|---|---|
| In methanol at 20℃; | 100% |
-
-
109-94-4
formic acid ethyl ester

-
-
502-42-1
cycloheptanone

-
-
64799-08-2, 1589-24-8
2-formylcycloheptanone

| Conditions | Yield |
|---|---|
| With ethanol; sodium hydride In diethyl ether at 20℃; for 22h; | 99% |
| With diethyl ether; sodium methylate | |
| With sodium ethanolate |

| Conditions | Yield |
|---|---|
| Stage #1: furan With n-butyllithium; N,N,N,N,-tetramethylethylenediamine In tetrahydrofuran; hexanes at -78℃; for 1h; Inert atmosphere; Stage #2: cycloheptanone In tetrahydrofuran; hexanes at -78℃; for 1h; Inert atmosphere; Stage #3: With ammonium chloride In tetrahydrofuran; hexanes; water | 99% |
| With n-butyllithium; toluene-4-sulfonic acid Multistep reaction; | |
| With n-butyllithium In diethyl ether; hexane at 0℃; for 1h; | |
| Stage #1: furan With n-butyllithium In diethyl ether; hexane at 0℃; Stage #2: cycloheptanone In diethyl ether; hexane at 0 - 20℃; | |
| Stage #1: furan With n-butyllithium In tetrahydrofuran at -20 - 0℃; for 4h; Inert atmosphere; Stage #2: cycloheptanone In tetrahydrofuran at -78 - 20℃; for 6h; Inert atmosphere; |
-
-
109-77-3
malononitrile

-
-
502-42-1
cycloheptanone

-
-
23917-22-8
2-amino-5,6,7,8-tetrahydro-4H-cyclohepta[b]thiophene-3-carbonitrile

| Conditions | Yield |
|---|---|
| With morpholine; sulfur In ethanol at 70℃; for 17h; | 99% |
| With morpholine; sulfur In ethanol at 30℃; for 8h; Gewald Aminoheterocycles Synthesis; | 95% |
| With sulfur; L-proline In N,N-dimethyl-formamide at 60℃; for 10h; Gewald reaction; | 94% |
-
-
75-77-4
chloro-trimethyl-silane

-
-
502-42-1
cycloheptanone

-
-
22081-48-7
(cyclohept-1-en-1-yloxy)(trimethyl)silane

| Conditions | Yield |
|---|---|
| With magnesium In N,N-dimethyl-formamide at 15 - 25℃; | 99% |
| Stage #1: cycloheptanone With n-butyllithium In tetrahydrofuran at -78℃; Stage #2: chloro-trimethyl-silane In tetrahydrofuran at -78 - 20℃; Further stages.; | 99% |
| With 1,4-dioxane; lithium chloride; 2-mesitylmagnesium bromide In tetrahydrofuran at 0℃; for 1h; Inert atmosphere; | 96% |
Cycloheptanone Chemical Properties
Molecular Formula: C7H12O
Molecular Weight: 112.17
EINECS: 207-937-6
Melting point: -21 °C
Boiling Point: 180 °C at 760 mmHg
Flash Point: 55.6 °C
Index of Refraction: 1.449
Molar Refractivity: 32.41 cm3
Molar Volume: 120.6 cm3
Surface Tension: 31.5 dyne/cm
Density: 0.929 g/cm3
Enthalpy of Vaporization: 41.63 kJ/mol
Vapour Pressure: 0.915 mmHg at 25°C
Storage temp.: Flammables area
Water Solubility: Insoluble in water
Appearance: Clear colorless to yellow liquid
Structure of Suberone (CAS NO.502-42-1):
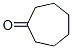
IUPAC Name: Cycloheptanone
Canonical SMILES: C1CCCC(=O)CC1
InChI: InChI=1S/C7H12O/c8-7-5-3-1-2-4-6-7/h1-6H2
InChIKey: CGZZMOTZOONQIA-UHFFFAOYSA-N
Product Category of Suberone (CAS NO.502-42-1): Pharmaceutical Intermediates;Carbonyl Compounds;Ketones
Cycloheptanone Uses
Suberone (CAS NO.502-42-1) is used in organic synthesis.
Cycloheptanone Toxicity Data With Reference
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LDLo | intraperitoneal | 750mg/kg (750mg/kg) | BEHAVIORAL: EXCITEMENT BEHAVIORAL: COMA CARDIAC: ARRHYTHMIAS (INCLUDING CHANGES IN CONDUCTION) | Comptes Rendus Hebdomadaires des Seances, Academie des Sciences. Vol. 254, Pg. 2245, 1962. |
| mouse | LDLo | subcutaneous | 930mg/kg (930mg/kg) | AUTONOMIC NERVOUS SYSTEM: OTHER (DIRECT) PARASYMPATHOMIMETIC BEHAVIORAL: COMA LUNGS, THORAX, OR RESPIRATION: RESPIRATORY DEPRESSION | Archiv fuer Experimentelle Pathologie und Pharmakologie. Vol. 50, Pg. 199, 1903. |
Cycloheptanone Safety Profile
Moderately toxic by subcutaneous and intraperitoneal routes. Can cause central nervous system depression. When heated to decomposition it emits acrid smoke and irritating fumes.
The Hazard Codes:  Xi
Xi
Hazard Note: Irritant
HazardClass: 3
The Risk Statements:
10: Flammable
36/37/38: Irritating to eyes, respiratory system and skin
The Safety Statements:
23: Do not breathe gas/fumes/vapor/spray (appropriate wording to be specified by the manufacturer)
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
24/25: Avoid contact with skin and eyes
RIDADR: UN 1987 3/PG 3
WGK Germany: 3
RTECS: GU3325000
PackingGroup: III
Cycloheptanone Specification
Suberone , its cas register number is 502-42-1. It also can be called Cycloheptanone ; Ketoheptamethylene ; and Suberon . It is hazardous, so the first aid measures and others should be known. Such as: When on the skin: first, should flush skin with plenty of water immediately for at least 15 minutes while removing contaminated clothing. Secondly, get medical aid. Or in the eyes: Flush eyes with plenty of water for at least 15 minutes, occasionally lifting the upper and lower eyelids. Then get medical aid soon. While, it's inhaled: Remove from exposure and move to fresh air immediately. Give artificial respiration while not breathing. When breathing is difficult, give oxygen. And as soon as to get medical aid. Then you have the ingesting of the product: Wash mouth out with water, and get medical aid immediately. Notes to physician: Treat supportively and symptomatically.
In addition, Suberone (CAS NO.502-42-1) is not compatible with strong oxidizing agents, reducing agents, strong bases, and you must not take it with incompatible materials, ignition sources. And also prevent it to broken down into hazardous decomposition products: Carbon monoxide, carbon dioxide.
Related Products
- Cycloheptanone
- Cycloheptanone,2-phenyl-
- 5024-21-5
- 502-44-3
- 502457-69-4
- 502457-70-7
- 502-47-6
- 50-24-8
- 502496-23-3
- 502496-24-4
- 502496-27-7
- 502496-33-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View