-
Name
D-Pantothenic acid
- EINECS 205-278-9
- CAS No. 79-83-4
- Article Data100
- CAS DataBase
- Density 1.266 g/cm3
- Solubility
- Melting Point 178-179 °C
- Formula C9H17NO5
- Boiling Point 551.505 °C at 760 mmHg
- Molecular Weight 219.238
- Flash Point 287.34 °C
- Transport Information
- Appearance
- Safety
- Risk Codes
-
Molecular Structure
- Hazard Symbols
- Synonyms Pantothenicacid, D- (8CI);b-Alanine,N-(2,4-dihydroxy-3,3-dimethyl-1-oxobutyl)-, (R)-;(+)-Pantothenic acid;(D)-(+)-Pantothenic acid;Chick antidermatitis factor;D(+)-N-(2,4-Dihydroxy-3,3-dimethylbutyryl)-b-alanine;β-Alanine, N-[(2R)-2,4-dihydroxy-3,3-dimethyl-1-oxobutyl]-;Pantothenic acid;Vitamin B3;Vitamin B5;
- PSA 106.86000
- LogP -0.65240
Synthetic route

| Conditions | Yield |
|---|---|
| With oxalic acid In water at 20℃; for 2h; | 95.11% |
| With (1S)-10-camphorsulfonic acid; trifluoroacetic acid at 0℃; | |
| With Amberlite IR-120 In water Inert atmosphere; | |
| With oxalic acid In water at 20℃; for 2h; |

| Conditions | Yield |
|---|---|
| With water at 20℃; | |
| With methanol at 20℃; | |
| With isopropyl alcohol bei Siedetemperatur; |
-
-
1265205-80-8
(R,E)-benzyl 3-(2,4-dihydroxy-3,3-dimethylbutanamido)acrylate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol for 24h; Inert atmosphere; | 86% |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With Cinchonidin | |
| With Quinine | |
| With quinine methohydroxide | |
| With quinine methohydroxide | |
| With Quinine |

| Conditions | Yield |
|---|---|
| With pantothenate synthetase; aspartate-α-decarboxylase; 3-methylaspartate ammonia lyase; ammonium chloride; ATP; magnesium chloride In aq. buffer at 25℃; for 24h; pH=9; Enzymatic reaction; | 70% |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With thionyl chloride Umsetzung des gebildeten Saeurechlorids mit 3-Amino-propionsaeure-aethylester in Pyridin und Verseifung des Reaktionsprodukts mit aethanol.NaOH; | |
| With thionyl chloride Umsetzung des gebildeten Saeurechlorids mit 3-Amino-propionsaeure-aethylester in Pyridin und Verseifung des Reaktionsprodukts mit wss.Ba(OH)2; |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With palladium 10% on activated carbon; hydrogen In methanol for 24h; | 92% |

| Conditions | Yield |
|---|---|
| at 70℃; Hydrolyse des Reaktionsprodukts mit 0.5n-Ba(OH)2 bei 20grad; | |
| at 70℃; Hydrolyse des Reaktionsprodukts mit 0.5n-Ba(OH)2 bei 20grad; |

| Conditions | Yield |
|---|---|
| durch Escherichia coli; | |
| durch Diphtherie-Bazillen; | |
| durch verschiedene Hefen; |

| Conditions | Yield |
|---|---|
| at 180℃; |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1: benzene / 19 h / 95 °C / Inert atmosphere 2: bismuth(III) chloride / water; acetonitrile / 3 h / 20 °C / Inert atmosphere 3: palladium 10% on activated carbon; hydrogen / methanol / 24 h / Inert atmosphere View Scheme |
-
-
1265205-79-5
(R,E)-benzyl 3-(2,2,5,5-tetramethyl-1,3-dioxane-4-carboxamido)acrylate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: bismuth(III) chloride / water; acetonitrile / 3 h / 20 °C / Inert atmosphere 2: palladium 10% on activated carbon; hydrogen / methanol / 24 h / Inert atmosphere View Scheme |
-
-
27778-35-4
(R)-2,4-Dihydroxy-3,3-dimethylbutyramide

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: pyridinium p-toluenesulfonate / Inert atmosphere 2.1: n-butyllithium / tetrahydrofuran; hexane / 0.08 h / 0 °C / Inert atmosphere 2.2: 0 - 20 °C / Inert atmosphere 3.1: benzene / 19 h / 95 °C / Inert atmosphere 4.1: bismuth(III) chloride / water; acetonitrile / 3 h / 20 °C / Inert atmosphere 5.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: ammonia / Inert atmosphere 2.1: pyridinium p-toluenesulfonate / Inert atmosphere 3.1: n-butyllithium / tetrahydrofuran; hexane / 0.08 h / 0 °C / Inert atmosphere 3.2: 0 - 20 °C / Inert atmosphere 4.1: benzene / 19 h / 95 °C / Inert atmosphere 5.1: bismuth(III) chloride / water; acetonitrile / 3 h / 20 °C / Inert atmosphere 6.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h / Inert atmosphere View Scheme |
-
-
285141-00-6
(R)-2,2,5,5-Tetramethyl-1,3-dioxane-4-carboxamide

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: n-butyllithium / tetrahydrofuran; hexane / 0.08 h / 0 °C / Inert atmosphere 1.2: 0 - 20 °C / Inert atmosphere 2.1: benzene / 19 h / 95 °C / Inert atmosphere 3.1: bismuth(III) chloride / water; acetonitrile / 3 h / 20 °C / Inert atmosphere 4.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h / Inert atmosphere View Scheme |

| Conditions | Yield |
|---|---|
| With Amberlite IR-120 (H+-form) In water | |
| With Dowex 50x8 (H+) In water |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With pantetheinase; water Enzymatic reaction; |

| Conditions | Yield |
|---|---|
| unter Einw. von Proteus morganii; | |
| unter Einw. von Clostridium septicum; | |
| unter Einw. von einem Stamm haemolytischer Streptococcen; |

| Conditions | Yield |
|---|---|
| unter Einw. von Proteus morganii; | |
| unter Einw. von einem Stamm haemolytischer Streptococcen; | |
| unter Einw. von Clostridium septicum; |

| Conditions | Yield |
|---|---|
| With pantothenase UK-1 at 25℃; Equilibrium constant; | |
| With 2-methoxy-ethanol; calcium | |
| at 155 - 180℃; |

| Conditions | Yield |
|---|---|
| With barium permanganate; sulfuric acid | |
| im Organismus von Ratten; |
-
-
895532-69-1
(R)-3-(benzyloxy)-3-[(R)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethylpropan-1-ol

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 7 steps 1.1: dmap; triethylamine / dichloromethane / 0 °C 1.2: 20 °C 2.1: acetic acid / 20 °C 3.1: sodium periodate / methanol; water 4.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 5.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 5.2: 16 h / 20 °C 6.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 7.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-61-4
(R)-3-(benzyloxy)-3-[(R)-2,2-dimethyl-1,3-dioxolan-4-yl]-2,2-dimethylpropyl acetate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 6 steps 1.1: acetic acid / 20 °C 2.1: sodium periodate / methanol; water 3.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 4.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 4.2: 16 h / 20 °C 5.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 6.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-62-5
(3R,4R)-3-(benzyloxy)-4,5-dihydroxy-2,2-dimethylpentyl acetate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 5 steps 1.1: sodium periodate / methanol; water 2.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 3.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 3.2: 16 h / 20 °C 4.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 5.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-63-6
(R)-3-(benzyloxy)-2,2-dimethyl-4-oxobutyl acetate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 4 steps 1.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 2.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 2.2: 16 h / 20 °C 3.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 4.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-64-7
(2R)-4-acetoxy-2-(benzyloxy)-3,3-dimethylbutanoic acid

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 3 steps 1.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 1.2: 16 h / 20 °C 2.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 3.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-65-8
methyl 3-[(R)-4-acetoxy-2-(benzyloxy)-3,3-dimethylbutanoylamino]propanoate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 2: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 11 steps 1.1: Dess-Martin periodane / dichloromethane / 4 h / 20 °C 2.1: sodium tetrahydroborate / methanol / 1 h / -40 °C 3.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0 °C 3.2: 20 °C 4.1: lithium aluminium tetrahydride / diethyl ether / 0 °C 5.1: dmap; triethylamine / dichloromethane / 0 °C 5.2: 20 °C 6.1: acetic acid / 20 °C 7.1: sodium periodate / methanol; water 8.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 9.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 9.2: 16 h / 20 °C 10.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 11.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-59-0
(R)-3-[(R)-2,2-dimethyl-1,3-dioxolan-4-yl]-3-hydroxy-2,2-dimethylpropanoate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 9 steps 1.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0 °C 1.2: 20 °C 2.1: lithium aluminium tetrahydride / diethyl ether / 0 °C 3.1: dmap; triethylamine / dichloromethane / 0 °C 3.2: 20 °C 4.1: acetic acid / 20 °C 5.1: sodium periodate / methanol; water 6.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 7.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 7.2: 16 h / 20 °C 8.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 9.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
1377411-50-1
ethyl (R)-3-(2,2-dimethyl-1,3-dioxolan-4-yl)-2,2-dimethyl-3-oxopropanoate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| Multi-step reaction with 10 steps 1.1: sodium tetrahydroborate / methanol / 1 h / -40 °C 2.1: sodium hydride / N,N-dimethyl-formamide; mineral oil / 0 °C 2.2: 20 °C 3.1: lithium aluminium tetrahydride / diethyl ether / 0 °C 4.1: dmap; triethylamine / dichloromethane / 0 °C 4.2: 20 °C 5.1: acetic acid / 20 °C 6.1: sodium periodate / methanol; water 7.1: sodium dihydrogenphosphate; sodium chlorite / methanol; dichloromethane; water / 20 °C 8.1: benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride / dichloromethane / 0 °C 8.2: 16 h / 20 °C 9.1: lithium hydroxide monohydrate / tetrahydrofuran; methanol; water / 3 h / 0 - 20 °C 10.1: palladium 10% on activated carbon; hydrogen / methanol / 24 h View Scheme |
-
-
79-83-4
pantothenic acid

-
-
108-24-7
acetic anhydride

-
-
96199-41-6
N-[(2R)-2,4-diacetoxy-3,3-dimethylbutanoyl]-β-alanine

| Conditions | Yield |
|---|---|
| With iodine at 0 - 20℃; | 97% |
-
-
79-83-4
pantothenic acid

-
-
116-11-0
2-Methoxypropene

-
-
167308-62-5
3-{[(4R)-2,2,5,5-tetramethyl-1,3-dioxan-4-yl]carbonylamino}propionic acid

| Conditions | Yield |
|---|---|
| With toluene-4-sulfonic acid In acetone at 0 - 20℃; for 0.666667h; | 95% |
| With toluene-4-sulfonic acid In acetone at 0 - 20℃; for 0.75h; Inert atmosphere; | |
| With toluene-4-sulfonic acid In acetone at 0 - 20℃; for 0.666667h; | 1.2 g |
-
-
107-10-8
propylamine

-
-
79-83-4
pantothenic acid

-
-
874304-32-2
(R)-2,4-dihydroxy-3,3-dimethyl-N-(3-oxo-3-(propylamino)propyl)butanamide

| Conditions | Yield |
|---|---|
| Stage #1: pantothenic acid With diphenyl phosphoryl azide; triethylamine In N,N-dimethyl-formamide at 0℃; for 0.333333h; Inert atmosphere; Stage #2: propylamine In N,N-dimethyl-formamide at 20℃; for 18h; Inert atmosphere; | 79% |
-
-
79-83-4
pantothenic acid

-
-
2186-92-7
p-Anisaldehyde dimethyl acetal

-
-
864239-47-4
3-{[(4R)-2-(p-methoxyphenyl)-5,5-dimethyl-1,3-dioxan-4-yl]carbonylamino}propionic acid

| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid In N,N-dimethyl-formamide at 20℃; for 16h; | 75% |
| With camphor-10-sulfonic acid In N,N-dimethyl-formamide | 68% |
| With (1S)-10-camphorsulfonic acid In dichloromethane at 20℃; | 51% |
| In dichloromethane at 20℃; for 8h; | 48% |
| With camphor-10-sulfonic acid In dichloromethane at 20℃; | 35% |
-
-
79-83-4
pantothenic acid

-
-
101759-90-4
N-(2-aminoethyl)-3-hydroxy-5,5-dimethoxy-3-methylpentanamide

-
-
101759-91-5, 101759-92-6
N-<3-<<(2,4-dihydroxy-3,3-dimethylbutanoyl)amino>propanamido>ethyl>-3-hydroxy-5,5-dimethoxy-3-methylpentanamide

| Conditions | Yield |
|---|---|
| With 2,2'-dipyridyldisulphide; triphenylphosphine In N,N-dimethyl-formamide for 1h; | 71% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 24h; | 68% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 24h; | 68% |
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; triethylamine In acetonitrile at 20℃; for 24h; | 68% |
-
-
79-83-4
pantothenic acid

-
-
76-26-6, 3144-16-9, 5872-08-2, 35963-20-3
camphor-10-sulfonic acid

| Conditions | Yield |
|---|---|
| In 4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran | 51% |


| Conditions | Yield |
|---|---|
| With camphor-10-sulfonic acid at 20℃; for 16h; Inert atmosphere; | 50% |

| Conditions | Yield |
|---|---|
| With diethyl cyanophosphonate; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.16667h; | 48% |
| With diphenyl phosphoryl azide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 3.16667h; | 42% |

| Conditions | Yield |
|---|---|
| With diphenylphosphoranyl azide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.33333h; | 46% |
| With diphenylphosphoranyl azide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.16667h; | 32% |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With barium permanganate; sulfuric acid |

| Conditions | Yield |
|---|---|
| unter Einw. von Acetobacter suboxydans; |

| Conditions | Yield |
|---|---|
| Hydrolysis.enzymatische Hydrolyse; |

| Conditions | Yield |
|---|---|
| Mechanism; multistep reaction; also by using (2RS)-(3S)-4-(13C)-valine; biosynthesis of pantothenic acid; stereospecificity of the biological hydroxymethylation of 2-oxo-isovaleric acid; |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| unter Einw. von Acetobacter suboxydans; |
-
-
14529-00-1
benzyl 3-aminopropionate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| at 100℃; Hydrogenolyse des Reaktionsprodukts an Platin in Eisessig oder Ameisensaeure; |

| Conditions | Yield |
|---|---|
| With methanol |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With barium permanganate; sulfuric acid anschliessende Hydrolyse mit wss.Ba(OH)2; |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With dihydrogen peroxide; oxalic acid |

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| With barium dihydroxide; silver(l) oxide |
-
-
16690-93-0
sodium β-alaninate

-
-
79-83-4
pantothenic acid

| Conditions | Yield |
|---|---|
| at 100℃; |
-
-
144-62-7
oxalic acid

-
-
851986-98-6
(R)-N-(3,3-diethoxypropyl)-2,4-dihydroxy-3,3-dimethylbutanamide

-
-
79-83-4
pantothenic acid

-
-
79-83-4
pantothenic acid

-
-
538-75-0
dicyclohexyl-carbodiimide

-
-
75980-10-8
N-(D-Pantothenoyl)-N,N'-dicyclohexylurea

| Conditions | Yield |
|---|---|
| In 1,4-dioxane for 24h; | 42.3% |

| Conditions | Yield |
|---|---|
| With diphenylphosphoranyl azide; triethylamine In N,N-dimethyl-formamide at 0 - 20℃; for 2.16667h; | 39% |

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In N,N-dimethyl-formamide | 38% |

| Conditions | Yield |
|---|---|
| With hydrogenchloride | 33% |
-
-
619-99-8
3-ethylhexane

-
-
79-83-4
pantothenic acid

-
-
96042-30-7
4-(dicyanomethylene)-2-methyl-6-(p-dimethylaminostyryl)-4H-pyran

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; triethylamine In methanol; N,N-dimethyl-formamide | 26.4% |

-
-
79-83-4
pantothenic acid

-
-
18542-42-2
(2-aminoethyl)methylsulfide

| Conditions | Yield |
|---|---|
| With benzotriazol-1-ol; 1-ethyl-(3-(3-dimethylamino)propyl)-carbodiimide hydrochloride; N-ethyl-N,N-diisopropylamine In dichloromethane at 25℃; for 2h; | 21.02% |
D-Pantothenic acid Specification
The D-Pantothenic acid, with the CAS registry number 79-83-4, is also known as β-Alanine, N-[(2R)-2,4-dihydroxy-3,3-dimethyl-1-oxobutyl]-. It belongs to the product categories of Feed Additives; Aliphatics; Chiral Reagents; Intermediates & Fine Chemicals; Isotope Labelled Compounds; Pharmaceuticals. Its EINECS number is 205-278-9. This chemical's molecular formula is C9H17NO5 and molecular weight is 186.29. What's more, its systematic name is N-[(2R)-2,4-Dihydroxy-3,3-dimethylbutanoyl]-β-alanine. Its classification codes are: (1)Growth Substances; (2)Micronutrients; (3)Vitamin B Complex; (4)Vitamins. This chemical is a water-soluble vitamin. It is an essential nutrient for many animals. It is used in the synthesis of coenzyme A (CoA).
Physical properties of D-Pantothenic acid are: (1)ACD/LogP: -0.856; (2)# of Rule of 5 Violations: 0; (3)ACD/LogD (pH 5.5): -2.23; (4)ACD/LogD (pH 7.4): -3.99; (5)ACD/BCF (pH 5.5): 1.00; (6)ACD/BCF (pH 7.4): 1.00; (7)ACD/KOC (pH 5.5): 1.00; (8)ACD/KOC (pH 7.4): 1.00; (9)#H bond acceptors: 6; (10)#H bond donors: 4; (11)#Freely Rotating Bonds: 8; (12)Polar Surface Area: 106.86 Å2; (13)Index of Refraction: 1.512; (14)Molar Refractivity: 51.928 cm3; (15)Molar Volume: 173.094 cm3; (16)Polarizability: 20.586×10-24cm3; (17)Surface Tension: 54.81 dyne/cm; (18)Density: 1.267 g/cm3; (19)Flash Point: 287.34 °C; (20)Enthalpy of Vaporization: 95.614 kJ/mol; (21)Boiling Point: 551.505 °C at 760 mmHg; (22)Vapour Pressure: 0 mmHg at 25°C.
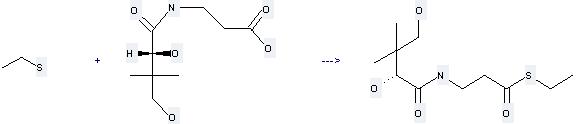
Uses of D-Pantothenic acid: it can be used to produce 3-(2,4-dihydroxy-3,3-dimethyl-butyrylamino)-thiopropionic acid S-ethyl ester at the temperature of 0 - 20 °C. It will need reagents diphenylphosphoryl azide, Et3N and solvent dimethylformamide with the reaction time of 140 min. The yield is about 46%.
You can still convert the following datas into molecular structure:
(1)SMILES: O=C(NCCC(=O)O)[C@H](O)C(C)(C)CO
(2)Std. InChI: InChI=1S/C9H17NO5/c1-9(2,5-11)7(14)8(15)10-4-3-6(12)13/h7,11,14H,3-5H2,1-2H3,(H,10,15)(H,12,13)/t7-/m0/s1
(3)Std. InChIKey: GHOKWGTUZJEAQD-ZETCQYMHSA-N
The toxicity data is as follows:
| Organism | Test Type | Route | Reported Dose (Normalized Dose) | Effect | Source |
|---|---|---|---|---|---|
| mouse | LD50 | intraperitoneal | 1443mg/kg (1443mg/kg) | BEHAVIORAL: ALTERED SLEEP TIME (INCLUDING CHANGE IN RIGHTING REFLEX) BEHAVIORAL: SOMNOLENCE (GENERAL DEPRESSED ACTIVITY) | Pharmaceutical Chemistry Journal Vol. 17, Pg. 32, 1983. |
| mouse | LD50 | subcutaneous | 2500mg/kg (2500mg/kg) | Journal of the American College of Toxicology. Vol. 6(1), Pg. 139, 1987. | |
| mouse | LD50 | unreported | 2490mg/kg (2490mg/kg) | Bitamin. Vol. 25, Pg. 297, 1962. | |
| rat | LD50 | subcutaneous | 3500mg/kg (3500mg/kg) | Journal of the American College of Toxicology. Vol. 6(1), Pg. 139, 1987. |
Related Products
- D-Pantothenic acid
- D-Pantothenic Acid Calcium Salt Hydrate
- 79836-44-5
- 79836-78-5
- 79839-49-9
- 79-84-5
- 79849-61-9
- 798544-32-8
- 79855-95-1
- 79-85-6
- 798-61-8
- 79864-22-5
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View