-
Name
Di-tert-Butyl azodicarboxylate
- EINECS 212-796-9
- CAS No. 870-50-8
- Article Data15
- CAS DataBase
- Density 1.06 g/cm3
- Solubility insoluble in water
- Melting Point 89-92 °C
- Formula C10H18N2O4
- Boiling Point 287.1 °C at 760 mmHg
- Molecular Weight 230.264
- Flash Point 107.2 °C
- Transport Information
- Appearance yellow crystals or crystalline powder
- Safety 26-36-37/39-16
- Risk Codes 36/37/38-11
-
Molecular Structure
-
Hazard Symbols
 Xi,
Xi,  F
F
- Synonyms Diazenedicarboxylicacid, bis(1,1-dimethylethyl) ester (9CI);Formic acid, azodi-, di-tert-butylester (7CI,8CI);Azodicarboxylic acid di-tert-butyl ester;Bis(1,1-dimethylethyl)azodicarboxylate;DBAD;1,2-Diazenedicarboxylicacid, 1,2-bis(1,1-dimethylethyl) ester;Di-tert-butyl azodiformate;Di-tert-butyl diazodicarboxylate;NSC 109889;
- PSA 77.32000
- LogP 3.30880
Synthetic route
-
-
16466-61-8
1,2-bis(t-butyloxycarbonyl)hydrazine

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| With pyridine; bromine In dichloromethane at 0℃; for 0.25h; | 100% |
| With pyridine; bromine In dichloromethane at 0℃; for 1.5h; Inert atmosphere; | 92% |
| With pyridine; bromine In dichloromethane at 0℃; for 0.75h; | 87% |
-
-
16466-61-8
1,2-bis(t-butyloxycarbonyl)hydrazine

-
-
28899-97-0
triphenylbismuth(V) diacetate

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 48h; |
-
-
24424-99-5
di-tert-butyl dicarbonate

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrazine hydrate / methanol / Inert atmosphere 2: pyridine; bromine / dichloromethane / Inert atmosphere View Scheme | |
| Multi-step reaction with 2 steps 1: hydrazine hydrate / methanol 2: pyridine; bromine / dichloromethane View Scheme | |
| Multi-step reaction with 2 steps 1: hydrazine hydrate / methanol / -10 - 20 °C 2: bromine; pyridine / dichloromethane / 0.5 h / 0 °C View Scheme |
-
-
24608-52-4
tert-butyl chloroformate

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| Multi-step reaction with 2 steps 1: hydrazine / ethanol; water / 0.5 h / 10 - 20 °C 2: pyridine; bromine / dichloromethane / 1.5 h / 0 °C / Inert atmosphere View Scheme |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
542-92-7
cyclopenta-1,3-diene

-
-
39203-22-0
di-tert-butyl 2,3-diazabicyclo[2.2.1]hept-5-ene-2,3-dicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 0℃; | 100% |
| In diethyl ether for 48h; Ambient temperature; | 94% |
| In dichloromethane Inert atmosphere; | 89% |
| In dichloromethane | 89% |
| In diethyl ether at 0℃; for 12h; |

| Conditions | Yield |
|---|---|
| With copper diacetate; 4 A molecular sieve In methanol at 40℃; for 0.666667h; | 100% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
603-33-8
triphenylbismuthane

-
-
65578-58-7
di-tert-butyl 1-phenylhydrazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| With copper diacetate; 1,1,1,3',3',3'-hexafluoro-propanol In acetonitrile at 75℃; for 0.25h; | 100% |
-
-
504-60-9
penta-1,3-diene

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1072150-92-5
di-tert-butyl 3-methyl-1,2,3,6-tetrahydropyridazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Diels-Alder reaction; | 100% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
7442-52-6, 125117-36-4
methyl 1-oxo-1,2,3,4-tetrahydronaphthalene-2-carboxylate

| Conditions | Yield |
|---|---|
| With 3-(3,5-bis(trifluoromethyl)anilino)-4-([(1R,2R)-2-(piperidin-1-yl)cyclohexyl]amino)cyclobut-3-ene-1,2-dione In toluene at -20℃; for 28h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 100% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
84109-76-2
tert-butyl 2-oxocyclopentanecarboxylate

| Conditions | Yield |
|---|---|
| With 3-(3,5-bis(trifluoromethyl)anilino)-4-([(1R,2R)-2-(piperidin-1-yl)cyclohexyl]amino)cyclobut-3-ene-1,2-dione In toluene at 20℃; for 17h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 100% |

| Conditions | Yield |
|---|---|
| With 2-[{(1R,2R)-2-(dimethylamino)cyclohexyl}amino]-8-fluoroquinazolin-4(1H)-one In toluene at -78℃; for 3h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 100% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1618700-81-4
2-but-3-enyl-2-(N, N'-di-tert-butoxycarbonylhydrazino)malonic acid di-benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Inert atmosphere; | 100% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
173541-54-3
dibenzyl 2-allylmalonate

-
-
1618700-79-0
2-allyl-2-(N,N’-di-tert-butoxycarbonylhydrazino)malonic acid di-benzyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Inert atmosphere; | 100% |

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1618700-80-3
2-but-3-enyl-2-(N, N'-di-tert-butoxycarbonylhydrazino)malonic acid di-tert-butyl ester

| Conditions | Yield |
|---|---|
| With sodium hydride In tetrahydrofuran at 20℃; for 0.25h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| In acetonitrile at 20℃; for 2h; Inert atmosphere; | 100% |

| Conditions | Yield |
|---|---|
| Stage #1: 8-quinolinol With sodium hydride In tetrahydrofuran Inert atmosphere; Stage #2: di-tert-butyl-diazodicarboxylate In tetrahydrofuran at -5 - 60℃; for 1.5h; Reagent/catalyst; Solvent; Time; Temperature; Inert atmosphere; | 99.9% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
87895-02-1
7-methoxy-2,2-dimethyl-4-vinyl-2H-chromene

-
-
95334-16-0
3,4-bis(t-butoxycarbonyl)-8-methoxy-5,5-dimethyl-2,3,4,4a-tetrahydro-5H-chromeno<3,4-c>pyridazine

| Conditions | Yield |
|---|---|
| In dichloromethane for 1h; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
156699-37-5
(S)-4-benzyl-3-(5-bromopentanoyl)oxazolidine-2-one

-
-
156699-38-6
(4S)-3-<(3S)-N,N'-bis(t-butoxycarbonyl)hexahydropyridazine-3-carboxy>-4-phenylmethyl-2-oxazolidinone

| Conditions | Yield |
|---|---|
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; lithium diisopropyl amide at -78℃; | 99% |
| Stage #1: (S)-4-benzyl-3-(5-bromopentanoyl)oxazolidine-2-one With n-butyllithium; diisopropylamine In tetrahydrofuran; hexane at -78℃; for 0.583333h; Inert atmosphere; Stage #2: di-tert-butyl-diazodicarboxylate In tetrahydrofuran; hexane; dichloromethane at -78℃; for 4h; Inert atmosphere; Stage #3: With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone In tetrahydrofuran; hexane; dichloromethane at -78 - 20℃; Inert atmosphere; | 63% |
| Multistep reaction; | |
| With 1,3-dimethyl-3,4,5,6-tetrahydro-2(1H)-pyrimidinone; tetra-(n-butyl)ammonium iodide; lithium diisopropyl amide 1.) THF, -78 deg C, 2.) CH2Cl2; Yield given; Multistep reaction; |

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 16h; Diels-Alder reaction; | 99% |
| In dichloromethane at 20℃; for 12h; | 95% |
| In diethyl ether at 0℃; |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
592-46-1
hexa-2,4-diene

-
-
1072150-91-4
di-tert-butyl cis-3,6-dimethyl-1,2,3,6-tetrahydropyridazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; Diels-Alder reaction; | 99% |
| In tetrachloromethane at -40 - 20℃; for 170h; Diels-Alder reaction; | 65% |

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: 3-ethoxycarbonylpyrrolidin-2,5-dione With lanthanum(III) isopropoxide; N,N-dimethyl acetamide In chloroform at 0 - 20℃; Inert atmosphere; Stage #2: di-tert-butyl-diazodicarboxylate With air In chloroform at 0℃; for 1.5h; Inert atmosphere; optical yield given as %ee; enantioselective reaction; | 99% |
| With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester In ethyl acetate at -40℃; for 24h; Diels amination; optical yield given as %ee; | 99 %Spectr. |
| Stage #1: 3-ethoxycarbonylpyrrolidin-2,5-dione With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester In ethyl acetate at 0℃; for 1h; Inert atmosphere; Large scale; Stage #2: di-tert-butyl-diazodicarboxylate In ethyl acetate at 0 - 5℃; for 1h; Reagent/catalyst; Inert atmosphere; Large scale; enantioselective reaction; | n/a |
-
-
1109280-48-9
1,3-di(tert-butoxycarbonyl)succinimide

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -20℃; for 40h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
84109-76-2
tert-butyl 2-oxocyclopentanecarboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -40℃; for 16h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
375349-06-7
tert-butyl 2,3-dihydro-1-oxo-1H-indene-2-carboxylate

-
-
1068504-73-3
C24H34N2O7

| Conditions | Yield |
|---|---|
| With Br(1-)*C42H42P(1+); potassium carbonate In toluene at 0℃; for 0.5h; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
375349-06-7
tert-butyl 2,3-dihydro-1-oxo-1H-indene-2-carboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -20℃; for 14h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
3036-66-6
1-methoxybuta-1,3-diene

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
252984-42-2
di-tert-butyl 3-methoxy-1,2,3,6-tetrahydropyridazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| In dichloromethane at 20℃; for 2h; Diels-Alder reaction; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
916600-70-9
2,2,2-trifluoroethyl α-methyl α-cyanoacetate

-
-
1180556-64-2
C16H24F3N3O6

| Conditions | Yield |
|---|---|
| With 9-O-benzyl-6'-hydroxycinchonidine In toluene at -60℃; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
1109280-48-9
1,3-di(tert-butoxycarbonyl)succinimide

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -20℃; for 40h; optical yield given as %ee; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
84109-76-2
tert-butyl 2-oxocyclopentanecarboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -40℃; for 16h; optical yield given as %ee; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
375349-06-7
tert-butyl 2,3-dihydro-1-oxo-1H-indene-2-carboxylate

| Conditions | Yield |
|---|---|
| With dipotassium hydrogenphosphate; Br(1-)*C46H38F12P(1+) In toluene at -20℃; for 14h; optical yield given as %ee; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1190830-35-3
methyl 3-amino-2-(4-fluorophenyl)-3-oxopropanoate

-
-
1190830-46-6
(R)-di-tert-butyl 1-(1-amino-2-(4-fluorophenyl)-3-methoxy-1,3-dioxopropan-2-yl)hydrazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: methyl 3-amino-2-(4-fluorophenyl)-3-oxopropanoate With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester In ethyl acetate at 0 - 20℃; Stage #2: di-tert-butyl-diazodicarboxylate In ethyl acetate at 0℃; for 14h; optical yield given as %ee; enantioselective reaction; | 99% |
| With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester; triethylamine In ethyl acetate at 23℃; for 10h; Kinetics; Reagent/catalyst; Concentration; Temperature; optical yield given as %ee; enantioselective reaction; | > 99 %Spectr. |
-
-
1190830-38-6
ethyl 3-amino-2-(4-nitrophenyl)-3-oxopropanoate

-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1190830-49-9
(R)-di-tert-butyl 1-(1-amino-3-ethoxy-2-(4-nitrophenyl)-1,3-dioxopropan-2-yl)hydrazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 3-amino-2-(4-nitrophenyl)-3-oxopropanoate With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester In ethyl acetate at 0 - 20℃; Stage #2: di-tert-butyl-diazodicarboxylate In ethyl acetate at 0℃; for 10h; optical yield given as %ee; enantioselective reaction; | 99% |
-
-
870-50-8
di-tert-butyl-diazodicarboxylate

-
-
1190830-41-1
ethyl 3-amino-2-(naphthalen-2-yl)-3-oxopropanoate

-
-
1190830-52-4
(R)-di-tert-butyl 1-(1-amino-3-ethoxy-2-(naphthalen-2-yl)-1,3-dioxopropan-2-yl)hydrazine-1,2-dicarboxylate

| Conditions | Yield |
|---|---|
| Stage #1: ethyl 3-amino-2-(naphthalen-2-yl)-3-oxopropanoate With (R)-N-(2-hydroxyphenyl)-2-(2-hydroxybenzoylamino)-3-methylbutylamide; lanthanum(III) nitrate hexahydrate; D-valine t-butyl ester In ethyl acetate at 0 - 20℃; Stage #2: di-tert-butyl-diazodicarboxylate In ethyl acetate at 0℃; for 18h; optical yield given as %ee; enantioselective reaction; | 99% |
Di-tert-Butyl azodicarboxylate Chemical Properties
The Molecular formula of Di-tert-Butyl azodicarboxylate(870-50-8): C10H18N2O4
The Molecular Weight of Di-tert-Butyl azodicarboxylate(870-50-8): 230.26
The Molecular Structure of Di-tert-Butyl azodicarboxylate(870-50-8): 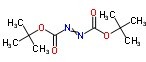
EINECS: 212-796-9
Melting point: 89-92 °C(lit.)
Boiling Point: 287.1 °C at 760 mmHg
Flash Point: 107.2 °C
Index of Refraction: 1.459
Molar Refractivity: 59.17 cm3
Molar Volume: 216.1 cm3
Polarizability: 23.45×10-24 cm3
Surface Tension: 32 dyne/cm
Density: 1.06 g/cm3
Enthalpy of Vaporization: 52.63 kJ/mol
Vapour Pressure: 0.00253 mmHg at 25°C
Storage temp.: 2-8°C
Water Solubility: Insoluble
Sensitive: Light Sensitive
BRN: 1911434
IUPAC Name: tert-butyl N-[(2-methylpropan-2-yl)oxycarbonylimino]carbamate
Synonyms: Azodicarboxylic acid di-tert-butyl ester;DI-T-BUTYL AZODICARBOXYLATE;Di-tert-butyl azodicarboxylate 98%;Di-tert-butyl Azodicarboxylate (20% in Toluene);DI-TERT-BUTYL AZODICARBOXYLATE;Di-tert-B-butyl azodicarboxylate, 98%;1,2-Bis(tert-butoxycarbonyl)diazene;Di-Tert-ButylAzodicarboxylate(Dbad);
Di-tert-Butyl azodicarboxylate Safety Profile
The Hazard Codes of Di-tert-Butyl azodicarboxylate(870-50-8):  Xi,
Xi,  F
F
Hazard Note: Irritant
The Hazard Codes of Di-tert-Butyl azodicarboxylate(870-50-8):
11: Highly Flammable
36/37/38: Irritating to eyes, respiratory system and skin
The Hazard Codes of Di-tert-Butyl azodicarboxylate(870-50-8):
16: Keep away from sources of ignition - No smoking
26: In case of contact with eyes, rinse immediately with plenty of water and seek medical advice
36: Wear suitable protective clothing
37/39: Wear suitable gloves and eye/face protection
RIDADR: 1325
WGK Germany: 3
Related Products
- Di-tert-Butyl 2,6-diazaspiro[3.3]heptane-2,6-dicarboxylate
- Di-tert-butyl 2,6-dimethyl-1,4-dihydropyridine-3,5-dicarboxylate
- Di-tert-Butyl azodicarboxylate
- Di-tert-butyl dicarbonate
- DI-tert-BUTYL DIPEROXYCARBONATE
- DI-tert-BUTYL DIPEROXYOXALATE
- Di-tert-butyl diperoxyphthalate
- Di-tert-butyl hydrazodiformate
- Di-tert-butyl iminodicarboxylate
- Di-tert-Butyl malonate
- 87051-43-2
- 870521-30-5
- 870521-31-6
- 870521-33-8
- 870521-57-6
- 870-55-3
- 87-05-8
- 87059-84-5
- 870606-45-4
- 87061-04-9
Hot Products
About|Contact|Cas|Product Name|Molecular|Country|Encyclopedia
Message|New Cas|MSDS|Service|Advertisement|CAS DataBase|Article Data|Manufacturers | Chemical Catalog
©2008 LookChem.com,License: ICP
NO.:Zhejiang16009103
complaints:service@lookchem.com Desktop View